First-in-human PET neuroimaging of [18F]OXD-2314
- PMID: 40553116
- PMCID: PMC12660345
- DOI: 10.1007/s00259-025-07413-w
First-in-human PET neuroimaging of [18F]OXD-2314
Abstract
Purpose: This first-in-human positron emission tomography (PET) study evaluates [18F]OXD-2314, a radiopharmaceutical designed for imaging tau in non-Alzheimer's disease tauopathies.
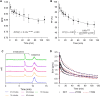
Methods: Synthesis of [18F]OXD-2314 was automated using a commercial module and validated for human use. Dynamic PET imaging was performed in healthy control subjects (2 female, 2 male, ages 49-65 years). Kinetic modelling was performed from brain time-activity curves and radiometabolite-corrected arterial input functions to estimate total distribution volumes (VT) in each region of interest.
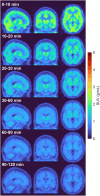
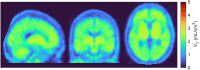
Results: [18F]OXD-2314 met all release criteria for human use. PET imaging revealed an initial whole brain peak of 2.3 standardized uptake value, followed by a steady washout. Distribution of radioactivity was uniform among brain regions (VT range: 2.21 ± 0.29 to 2.81 ± 0.43 mL/cm3).
Conclusions: [18F]OXD-2314 was successfully translated to first-in-human PET imaging. No adverse events were reported and PET imaging in patient populations of non-AD tauopathies is underway.
Keywords: Alzheimer’s disease; First-in-human; PET; Tau; [18F]OXD-2314.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was approved by the CAMH Research Ethics Board and was conducted in accordance with the Declaration of Helsinki ethical standards. Consent to participate: Participants provided written informed consent in compliance with the Tri-Council Policy Statement of Ethical Conduct for Research Involving Humans. Consent to publish: Not applicable. Competing interests: S.S. was an employee of Oxiant Discovery during the conduct of the reported study who may own or hold stock options in the company. N.V. is a co-founder of MedChem Imaging, Inc., which did not contribute support for this study. The authors declare no other competing interest.
Figures
References
-
- Wongso H, Harada R, Furumoto S. Current progress and future directions in non-Alzheimer’s disease tau PET tracers. ACS Chem Neurosci. 2025;16:111–27. 10.1021/acschemneuro.4c00319. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

