Automated three-dimensional computed tomography analysis for surgical decisions in congenital nasal pyriform aperture stenosis
- PMID: 40553166
- PMCID: PMC12321926
- DOI: 10.1007/s00247-025-06282-7
Automated three-dimensional computed tomography analysis for surgical decisions in congenital nasal pyriform aperture stenosis
Abstract
Background: Congenital nasal pyriform aperture stenosis is a rare neonatal condition that causes respiratory distress and potentially requires surgery. Current diagnosis relies on clinical assessment and manual computed tomography (CT) measurements of the pyriform aperture width, which may not fully capture obstruction severity.
Objective: To evaluate the severity of pyriform aperture stenosis using automatic three-dimensional (3-D) analysis and identify parameters discriminating between conservative and surgical cases.
Materials and methods: This retrospective study analyzed CT scans of neonatal airways using a novel automated 3-D segmentation algorithm. We collected 22 CT scans (2010-2022) of newborns aged 0-35 days: 12 controls, four moderate cases treated conservatively, and six severe cases requiring surgery. The algorithm measured pyriform aperture width, nasal volumes, surface area, and cross-sectional areas.
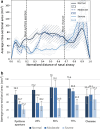
Results: The algorithm achieved high accuracy (Dice coefficient, 0.961 ± 0.005) and aligned well with manual measurements of the pyriform aperture (average difference, -0.05 ± 0.77 mm, -0.7 ± 9.1%). All cases with stenosis showed anterior narrowing, while only severe cases exhibited stenosis along the entire mid-nasal cavity. Mid-nasal cavity volume, surface area, and cross-sectional areas at 50% and 75% of the mid-nasal cavity emerged as potential surgical predictors, with cross-sectional area at 75% being the most discriminating (moderate, 68.6 ± 7.5 mm2; severe, 33.5 ± 13.7 mm2; P<0.01).
Conclusion: Automated 3-D CT analysis quantifies pyriform aperture stenosis severity by measuring nasal airway dimensions. The study suggests objective parameters that may assist in surgical decisions and highlights the importance of considering the entire 3-D nasal cavity when planning surgical interventions. A multi-center study with a larger cohort is recommended to validate these findings.
Keywords: Airway management; Computed tomography; Computer-assisted; Computer-assisted decision-making; Congenital nasal pyriform aperture stenosis; Image interpretation; Three-dimensional imaging.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Nasal cavity dimensions in congenital pyriform aperture stenosis.Int J Pediatr Otorhinolaryngol. 2013 Nov;77(11):1830-2. doi: 10.1016/j.ijporl.2013.08.021. Epub 2013 Aug 29. Int J Pediatr Otorhinolaryngol. 2013. PMID: 24035640
-
Congenital nasal pyriform aperture stenosis; our experience of 34 cases.Int J Pediatr Otorhinolaryngol. 2023 Mar;166:111491. doi: 10.1016/j.ijporl.2023.111491. Epub 2023 Feb 27. Int J Pediatr Otorhinolaryngol. 2023. PMID: 36870158
-
The Role of the Inferior Turbinates in Congenital Pyriform Aperture Stenosis.Ann Otol Rhinol Laryngol. 2025 Jun 19:34894251349648. doi: 10.1177/00034894251349648. Online ahead of print. Ann Otol Rhinol Laryngol. 2025. PMID: 40537407
-
Patient selection in congenital pyriform aperture stenosis repair - 14 year experience and systematic review of literature.Int J Pediatr Otorhinolaryngol. 2015 Feb;79(2):235-9. doi: 10.1016/j.ijporl.2014.12.016. Epub 2014 Dec 22. Int J Pediatr Otorhinolaryngol. 2015. PMID: 25575426
-
Congenital nasal pyriform aperture stenosis: a report of 10 cases and literature review.Int J Pediatr Otorhinolaryngol. 2012 Jan;76(1):28-30. doi: 10.1016/j.ijporl.2011.09.016. Epub 2011 Oct 22. Int J Pediatr Otorhinolaryngol. 2012. PMID: 22024577 Review.
References
-
- Brown OE, Myer CM, Manning SC (1989) Congenital nasal pyriform aperture stenosis. Laryngoscope 99:86–91. 10.1288/00005537-198901000-00016 - PubMed
-
- Belden CJ, Mancuso AA, Schmalfuss IM (1999) CT features of congenital nasal piriform aperture stenosis: initial experience. Radiology 213:495–500. 10.1148/RADIOLOGY.213.2.R99OC38495 - PubMed
-
- Wormald R, Hinton-Bayre A, Bumbak P, Vijayasekaran S (2015) Congenital nasal pyriform aperture stenosis 5.7mm or less is associated with surgical intervention: a pooled case series. Int J Pediatr Otorhinolaryngol 79:1802–1805. 10.1016/j.ijporl.2015.07.026 - PubMed
-
- Gnagi SH, Schraff SA (2013) Nasal obstruction in newborns. Pediatr Clin North Am 60:903–922. 10.1016/j.pcl.2013.04.007 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

