From Prediction to Prevention: The Intricacies of Islet Autoantibodies in Type 1 Diabetes
- PMID: 40553206
- PMCID: PMC12187867
- DOI: 10.1007/s11892-025-01595-1
From Prediction to Prevention: The Intricacies of Islet Autoantibodies in Type 1 Diabetes
Abstract
Purpose of review: This review synthesizes current knowledge on islet autoantibodies (IAs) as predictive biomarkers for type 1 diabetes (T1D), focusing on their role in disease staging, autoantibody patterns, advancements in screening methodologies, and the implications of implementing population-wide screening initiatives.
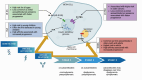
Recent findings: Autoantibody profiling has refined T1D risk stratification, with progression rates influenced by IA characteristics including number, type, order of appearance, and affinity. While screening efforts initially targeted genetically at-risk groups, approximately 90% of new TID diagnoses occur in individuals without a family history, underscoring the need for broader population-based screening. The approval of teplizumab, a therapy shown to delay clinical T1D onset, represents a paradigm shift by providing an intervention following early identification through screening. Technological advancements have further optimized IA detection and therapeutic strategies. However, challenges such as cost-effectiveness, implementation logistics, and assay standardization remain. T1D is a chronic autoimmune disorder characterized by progressive pancreatic beta-cell destruction, leading to insulin deficiency. The natural history of T1D is typically marked by the appearance of IAs long before clinical symptoms emerge, providing a window for early detection and intervention. Identifying at-risk individuals during this asymptomatic phase can reduce disease severity at clinical onset and facilitate timely application of disease-modifying therapies like teplizumab. Emerging evidence emphasizes that IA characteristics collectively shape disease risk and progression. Advancements in screening technologies and therapies continue to support the integration of IA screening into clinical care, marking a significant step toward effective T1D prevention and management.
Keywords: Autoantibodies; Screening; Type 1 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Z.Q. has acted as a scientific advisor to Sanofi for a scientific advisory board in November 2023. Competing interests : ZQ has previously been on a scientific advisory board for Sanofi. Consent: This article does not contain any studies with human or animal subjects performed by any of the authors. Human and Animal Rights and Informed Consent : This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures

Similar articles
-
Type 1 diabetes risk factors, risk prediction and presymptomatic detection: Evidence and guidance for screening.Diabetes Obes Metab. 2025 Aug;27 Suppl 6(Suppl 6):28-39. doi: 10.1111/dom.16354. Epub 2025 Mar 25. Diabetes Obes Metab. 2025. PMID: 40134221 Free PMC article. Review.
-
Establishing Screening Programs for Presymptomatic Type 1 Diabetes: Practical Guidance for Diabetes Care Providers.J Clin Endocrinol Metab. 2025 Jul 15;110(8):2371-2382. doi: 10.1210/clinem/dgaf194. J Clin Endocrinol Metab. 2025. PMID: 40171881 Free PMC article.
-
Increased inflammation as well as decreased endoplasmic reticulum stress and translation differentiate pancreatic islets from donors with pre-symptomatic stage 1 type 1 diabetes and non-diabetic donors.Diabetologia. 2025 Jul;68(7):1463-1475. doi: 10.1007/s00125-025-06417-3. Epub 2025 Jun 2. Diabetologia. 2025. PMID: 40457096 Free PMC article.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
References
-
- Gregory GA, Robinson TIG, Linklater SE, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10(10):741–60. 10.1016/S2213-8587(22)00218-2. - PubMed
-
- Thunander M, Petersson C, Jonzon K, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg Sweden. Diabetes Res Clin Pract. 2008;82(2):247–55. 10.1016/j.diabres.2008.07.022. - PubMed
-
- Karges B, Prinz N, Placzek K, et al. A Comparison of familial and sporadic type 1 diabetes among young patients. Diabetes Care. 2021;44(5):1116–24. 10.2337/dc20-1829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

