Nitrile Hydroboration by Cooperative Iron Catalysis: An Experimental and Computational Study
- PMID: 40553511
- PMCID: PMC12284615
- DOI: 10.1002/chem.202501782
Nitrile Hydroboration by Cooperative Iron Catalysis: An Experimental and Computational Study
Abstract
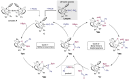
Reductive amination is a challenging reaction in catalysis that often gives poor yield and selectivity. We present an iron-catalyzed approach of synthesizing amines through reduction of nitriles through hydroboration with good yield under ambient conditions. Our detailed mechanistic study establishes the factors that influence the selectivity and turnover. The kinetics and mechanism of the iron-catalyzed hydroboration of benzonitrile to bis(boryl)benzylamine have been investigated by initial rates, temperature dependence, kinetic isotope effects, and computational studies. In contrast to other iron-catalyzed nitrile hydroboration, this study reveals that B─H bond activation is not rate-determining. Moreover, the rate-determining step was revealed to be C─H bond reductive elimination with an equilibrium isotope effect in operation. Through this combined approached, an Fe(0)/(II) catalytic manifold proceeding via metal-ligand cooperativity has been determined.
Keywords: DFT calculations; cooperative catalysis; hydroboration; iron catalysis; kinetic measurements.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Tejedor D., Cotos L., Méndez‐Abt G., García‐Tellado F., J. Org. Chem. 2014, 79, 10655. - PubMed
-
- Afanasyev O. I., Kuchuk E., Usanov D. L., Chusov D., Chem. Rev. 2019, 119, 11857. - PubMed
-
- Sharkey A. M., Hartig A. M., Dang A. J., Chatterjee A., Williams B. J., Parker K. M., Environ. Sci. Technol. 2022, 56, 13644. - PubMed
-
- Lubinu M. C., De Luca L., Giacomelli G., Porcheddu A., Chem. Eur. J. 2011, 17, 82. - PubMed
-
- Marzaro G., Guiotto A., Chilin A., Green Chem. 2009, 11, 774.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

