LRP2 is a potential molecular target for nonsyndromic pathological myopia
- PMID: 40553567
- PMCID: PMC12333944
- DOI: 10.1172/jci.insight.192929
LRP2 is a potential molecular target for nonsyndromic pathological myopia
Abstract
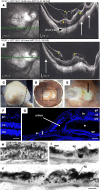
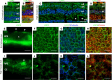
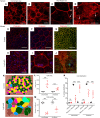
High myopia (HM) and posterior staphyloma (PS) are major causes of vision loss worldwide. Genetic and environmental factors, especially light exposure, influence myopia. This study shows that low-density lipoprotein-related receptor type 2 (LRP2) levels are decreased in the vitreous of patients with HM and PS, and that in human donor eyes affected by PS, LRP2 expression was reduced in the neural retina and retinal pigment epithelium (RPE), with morphologic changes similar to those observed in the Foxg1-Cre-Lrp2fl/fl mouse that also develops PS. In human induced pluripotent stem cell-derived RPE cells, LRP2 silencing regulated genes involved in eye and neuronal development, visual perception, tissue remodeling, hormone metabolism, and RPE structure. Its expression increased under light exposure, particularly red light, but was downregulated by cortisol. These findings establish a link between LRP2, myopization, and environmental factors, highlighting its crucial role in nonsyndromic HM and PS. LRP2 appears to be a promising therapeutic target for HM treatment.
Keywords: Neuroscience; Ophthalmology; Retinopathy.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

