Aphasia severity prediction using a multi-modal machine learning approach
- PMID: 40554033
- PMCID: PMC12261925
- DOI: 10.1016/j.neuroimage.2025.121300
Aphasia severity prediction using a multi-modal machine learning approach
Abstract
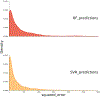
The present study examined an integrated multiple neuroimaging modality (T1 structural, Diffusion Tensor Imaging (DTI), and resting-state FMRI (rsFMRI)) to predict aphasia severity using Western Aphasia Battery-Revised Aphasia Quotient (WAB-R AQ) in 76 individuals with post-stroke aphasia. We employed Support Vector Regression (SVR) and Random Forest (RF) models with supervised feature selection and a stacked feature prediction approach. The SVR model outperformed RF, achieving an average root mean square error (RMSE) of 16.38±5.57, Pearson's correlation coefficient (r) of 0.70±0.13, and mean absolute error (MAE) of 12.67±3.27, compared to RF's RMSE of 18.41±4.34, r of 0.66±0.15, and MAE of 14.64±3.04. Resting-state neural activity and structural integrity emerged as crucial predictors of aphasia severity, appearing in the top 20% of predictor combinations for both SVR and RF. Finally, the feature selection method revealed that functional connectivity in both hemispheres and between homologous language areas is critical for predicting language outcomes in patients with aphasia. The statistically significant difference in performance between the model using only single modality and the optimal multi-modal SVR/RF model (which included both resting-state connectivity and structural information) underscores that aphasia severity is influenced by factors beyond lesion location and volume. These findings suggest that integrating multiple neuroimaging modalities enhances the prediction of language outcomes in aphasia beyond lesion characteristics alone, offering insights that could inform personalized rehabilitation strategies.
Keywords: Aphasia; Aphasia prediction; DTI; MRI; Machine learning; Multi-modal; fMRI.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Swathi Kiran reports a relationship with Constant Therapy Health that includes: equity or stocks. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




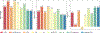
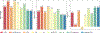





References
-
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. - PubMed
-
- Ashburner J, Friston KJ, 2005. Unified segmentation. Neuroimage 26, 839–851. - PubMed
-
- Awad M, Khanna R, 2015. Support vector regression, in: Efficient Learning Machines. Apress, Berkeley, CA, pp. 67–80.
-
- Billot A, Lai S, Varkanitsa M, Braun EJ, Rapp B, Parrish TB, Higgins J, Kurani AS, Caplan D, Thompson CK, Ishwar P, Betke M, Kiran S, 2022. Multimodal Neural and Behavioral Data Predict Response to Rehabilitation in Chronic Poststroke Aphasia. Stroke 53, 1606–1614. doi: 10.1161/STROKEAHA.121.036749. publisher: American Heart Association. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

