Multipopulation GWAS for venous thromboembolism identifies novel loci followed by experimental validation in zebrafish
- PMID: 40554366
- PMCID: PMC12495096
- DOI: 10.1182/bloodadvances.2024015790
Multipopulation GWAS for venous thromboembolism identifies novel loci followed by experimental validation in zebrafish
Abstract
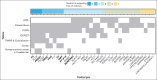
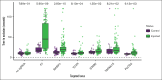
Venous thromboembolisms (VTEs) are a leading cause of morbidity and mortality. Although many genetic risk factors have been identified, a substantial portion of the heritability remains unexplained. In this study, we employed a genome-wide association study (GWAS) for VTE across 9 international cohorts of the Global Biobank Meta-Analysis Initiative to address this question, along with in vivo functional validation. In this multipopulation GWAS (VTE cases, 27 987; controls, 1 035 290), 38 genome-wide significant loci were identified, 4 of which were potentially novel. For each autosomal locus, we performed gene prioritization using 7 independent, yet converging, lines of evidence. Through prioritization, we identified genes associated with VTE through GWAS and/or functional studies (eg, F5, F11, VWF, STAB2, PLCG2, TC2N), functionally validated those that did not have evidence other than GWAS (TC2N, TSPAN15), and discovered 1 not previously associated with coagulation (RASIP1). We evaluated the function of 6 prioritized genes with strong genetic evidence, including F7 as a positive control, using laser-mediated endothelial injury to induce thrombosis in zebrafish after CRISPR/Cas9 knockdown. From this assay, we have supportive evidence for the role of RASIP1 and TC2N in the modification of human VTE and suggestive evidence for STAB2 and TSPAN15. This study expands on the currently identified genomic architecture of VTE through biobank-based, multipopulation GWASs, in silico candidate gene predictions, and in vivo functional follow-up of candidate genes.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.J.W. and K.-H.H.W. report being employed at Regeneron Pharmaceuticals, although they were not at the time of this study. D.K. reports being employed at Bitterroot Bio, although he was not at the time of this study. S.M.D. reports research support from Novo Nordisk and Amgen, outside the scope of the current research; and is named as a coinventor on a government-owned US Patent application related to the use of genetic risk prediction for venous thromboembolic disease filed by the US Department of Veterans Affairs in accordance with Federal regulatory requirements. J.A.S. reports serving as a consultant for Sanofi, Novo Nordisk, Biomarin, Takeda, Pfizer, Genentech, CSL Behring, and Medexus. The remaining authors declare no competing financial interests.
A complete list of the members of the Global Biobank Meta-analysis Initiative (GBMI) study group and INVENT, MVP consortium appears in the supplemental Appendix.
Figures


References
-
- Henke PK, Kahn SR, Pannucci CJ, et al. American Heart Association Advocacy Coordinating Committee Call to action to prevent venous thromboembolism in hospitalized patients: a policy statement from the American Heart Association. Circulation. 2020;141(24):e914–e931. - PubMed
-
- Centers for Disease Control and Prevention Data and statistics on venous thromboembolism. https://www.cdc.gov/blood-clots/data-research/facts-stats/index.html
-
- Centers for Disease Control and Prevention Impact of blood clots on the United States. https://www.cdc.gov/blood-clots/toolkit/impact-of-blood-clots.html?CDC_A...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

