Enzymatic Reactions Dictated by the 2D Membrane Environment
- PMID: 40554482
- PMCID: PMC12235624
- DOI: 10.1021/acs.jpclett.5c00988
Enzymatic Reactions Dictated by the 2D Membrane Environment
Abstract
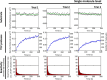
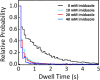
The cell membrane is a critical component of cellular architecture, serving not only as a physical barrier enclosing the cytosol but also as a dynamic platform for various biochemical reactions. Due to the unique two-dimensional and fluidic environment of the membrane, reactions that occur on its surface are subject to specific physical constraints. While membrane-mediated reactions are known to play key roles in cellular regulation, their advantages and limitations remain inadequately explored. In this study, we reconstitute a classic proteolytic cleavage reaction at the membrane interface, designed for the real-time kinetic analysis down to the single-molecule level. By systematically altering the enzyme-membrane affinity, we examined enzyme-substrate interactions under various conditions. Our findings reveal that while the membrane environment significantly enhances enzymatic turnover rate, it also imposes diffusion limitations that immediately reduce this turnover rate over time. By adjusting the enzyme's membrane affinity to an intermediate level, we enable the enzyme to "hop" on the membrane surface, overcoming these diffusion constraints and sustaining high enzymatic turnover rate with faster kinetics. These results highlight the dual role of the membrane environment in regulating biochemical reactions, balancing enhanced reactivity with physical limitations. Moreover, the ability to dynamically tune membrane affinity to optimize reactions underscores the cell's capacity to regulate enzymatic processes efficiently. This study provides critical insights into the role of the cell membrane in biochemical reactions and offers a broadly applicable framework for understanding membrane-associated interactions in biological systems.
Figures




References
-
- Lim, W. ; Mayer, B. ; Pawson, T. . Cell Signaling: Principles and Mechanisms; Garland Science, 2015.
MeSH terms
LinkOut - more resources
Full Text Sources

