The in cellular and in vivo melanogenesis inhibitory activity of safflospermidines from Helianthus annuus L. bee pollen in B16F10 murine melanoma cells and zebrafish embryos
- PMID: 40554513
- PMCID: PMC12186964
- DOI: 10.1371/journal.pone.0325264
The in cellular and in vivo melanogenesis inhibitory activity of safflospermidines from Helianthus annuus L. bee pollen in B16F10 murine melanoma cells and zebrafish embryos
Abstract
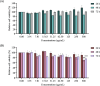
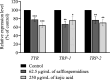
Melanin, synthesized by tyrosinase (TYR), is a natural pigment essential for skin protection and pigmentation. However, excessive melanin production can cause dermatological disorders. Safflospermidines, comprised of safflospermidine A and B isomers isolated from sunflower (Helianthus annuus L.) bee pollen, were shown to exhibit a strong in vitro TYR inhibitory activity against mushroom TYR. However, their anti-melanogenesis activity in cellular and in vivo models remains unexplored. This study firstly evaluated the effects of these safflospermidines on melanogenesis in α-melanocyte stimulating hormone-stimulated B16F10 cells, using kojic acid as a positive control. Cytotoxicity was evaluated using the MTT assay, while TYR activity and melanin content were measured to assess melanogenesis inhibition. The expression of key melanogenesis-related genes was analyzed through quantitative real-time reverse transcription (RT-q)PCR to elucidate the molecular mechanisms involved. Secondly, the melanogenic activity and potential toxicity of the compounds were confirmed in zebrafish embryos, with phenylthiourea (PTU) as a reference. The results revealed that a mixture of these two safflospermidines exhibited no cytotoxicity across the treated concentration range (0-500 µg/mL). At 62.5 µg/mL, safflospermidines significantly reduced the intracellular melanin content by 21.78 ± 4.01% and the TYR activity by 25.71 ± 3.08% in B16F10 cells through downregulation of TYR, TYR-related protein 1 (TRP-1), and TRP-2 gene expression. Additionally, the safflospermidines induced a noticeable reduction in dendritic cell structures, which likely contributed to the marked decrease in extracellular melanin levels. Kojic acid (250 μg/mL) significantly reduced the melanin content and TYR activity by suppressing all three melanogenesis-related genes. In zebrafish embryos, safflospermidines showed no toxicity or morphological abnormalities within a concentration range of 0-62.5 µg/mL, and at a concentration of 15.63 µg/mL the melanin production in zebrafish embryos was significantly reduced by 28.43 ± 9.17%. In comparison, 0.0015% (v/v) PTU decreased melanin production by 53.20 ± 3.75%. These findings suggest that safflospermidines are safe, effective melanin inhibitors with potential applications in pharmaceuticals and cosmetics for managing hyperpigmentation.
Copyright: © 2025 Khongkarat et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
No authors have competing interests.
Figures





Similar articles
-
Inhibitory activity of soybean (Glycine max L. Merr.) Cell Culture Extract on tyrosinase activity and melanin formation in alpha-melanocyte stimulating Hormone-Induced B16-F10 melanoma cells.Mol Biol Rep. 2022 Aug;49(8):7827-7836. doi: 10.1007/s11033-022-07608-6. Epub 2022 Jun 22. Mol Biol Rep. 2022. PMID: 35733058
-
Anti-Melanogenesis Activity of Peptides from Shark (Mustelus griseus) Skin on B16F10 Melanocytes and In vivo Zebrafish Models.Appl Biochem Biotechnol. 2025 Aug;197(8):5396-5410. doi: 10.1007/s12010-025-05296-z. Epub 2025 Jun 17. Appl Biochem Biotechnol. 2025. PMID: 40526244
-
Inhibition of Tyrosinase and Melanogenesis by a White Mulberry Fruit Extract.Int J Mol Sci. 2025 Aug 6;26(15):7589. doi: 10.3390/ijms26157589. Int J Mol Sci. 2025. PMID: 40806718 Free PMC article.
-
The melanin inhibitory effect of plants and phytochemicals: A systematic review.Phytomedicine. 2022 Dec;107:154449. doi: 10.1016/j.phymed.2022.154449. Epub 2022 Sep 6. Phytomedicine. 2022. PMID: 36126406
-
Natural sources of melanogenic inhibitors: A systematic review.Int J Cosmet Sci. 2022 Apr;44(2):143-153. doi: 10.1111/ics.12763. Epub 2022 Feb 22. Int J Cosmet Sci. 2022. PMID: 35048395
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

