Assessment of a multiplex arbovirus PCR Detection Test in an area endemic for Chikungunya, Zika, and Dengue viruses: An evaluation of kit performance characteristics in line with Clinical Laboratory Improvement Amendments (CLIA) Standards
- PMID: 40554518
- PMCID: PMC12186890
- DOI: 10.1371/journal.pone.0309626
Assessment of a multiplex arbovirus PCR Detection Test in an area endemic for Chikungunya, Zika, and Dengue viruses: An evaluation of kit performance characteristics in line with Clinical Laboratory Improvement Amendments (CLIA) Standards
Abstract
Introduction: Several multiplex and singleplex PCR systems exist for the detection of Dengue(DENV), Zika(ZIKV), and Chikungunya(CHIKV) viruses; however, their performance characteristics in East Africa remain unassessed. In this study, we investigated the TaqMan® Arbovirus Triplex Kit, which allows for the simultaneous identification of CHIKV, DENV, and ZIKV viruses in serum samples and singleplex assays, including the RealStar® Chikungunya RT-PCR Kit 2.0, RealStar® Dengue RT-PCR Kit 3.0, and RealStar® Zika Virus RT-PCR Kit 1.0.
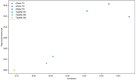
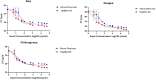
Methods: We used Clinical Laboratory Improvement validation methods and residual specimens sourced from DENV, CHIKV, and ZIKV outbreaks, from Kenya, Brazil, and the Democratic Republic of Congo(DRC) in March 2024. Quality control was ensured through Quality Control for Medical diagnostics (QCMD) commercial positive panels. Gold standard results were established through consensus from multiple kits, including the TaqMan® Arbovirus Triplex Kit, Illumina viral surveillance panel (Illumina VSP), and Altona singleplex kit. Data were analyzed using R to determine specificity, sensitivity, negative predictive value (NPV), positive predictive value (PPV), and diagnostic odds ratio, with probit analysis employed to evaluate the limit of detection.
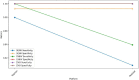
Results: The TaqMan® Arbovirus Kit showed sensitivity exceeding 95% for all three virus targets: 97.98% for DENV, and 100% for both CHIKV and ZIKV. Simillarly, the RealStar® RT-PCR Kits exhibited sensitivities: 93.94% for CHIKV, 90.91% for DENV, and 100% for ZIKV. All kits demonstrated specificity above 99%, with a positive predictive value (PPV) over 99% and a negative predictive value (NPV) exceeding 97%. Probit analysis revealed that the TaqMan® Arbovirus Kit also had a lower detection limit compared to the RealStar kit for all tested viruses.
Conclusion: The TaqMan® Arbovirus Kit provides multiplex capabilities and performance similar to the RealStar® CHIKV, DENV, and ZIKV RT-PCR Kits. Consequently, these kits can be used interchangeably for detecting DENV, CHIKV, and ZIKV viruses in outbreak situations.
Copyright: © 2025 Kingwara et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
No authors have competing interests.
Figures
References
-
- Wu W, Wang J, Yu N, Yan J, Zhuo Z, Chen M. Development of multiplex real-time reverse-transcriptase polymerase chain reaction assay for simultaneous detection of Zika, dengue, yellow fever, and chikungunya viruses in a single tube. J Med Virol. 2018;90(11). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical