Tomato Spotted Wilt Virus Promotes Offspring and Egg Production of Its Vector, Frankliniella occidentalis, by Suppressing Plant Defences Induced by a Thrips Salivary Elicitor
- PMID: 40554657
- PMCID: PMC12186865
- DOI: 10.1111/mpp.70112
Tomato Spotted Wilt Virus Promotes Offspring and Egg Production of Its Vector, Frankliniella occidentalis, by Suppressing Plant Defences Induced by a Thrips Salivary Elicitor
Abstract
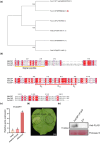
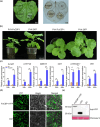
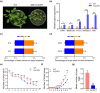
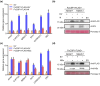
Western flower thrips (Frankliniella occidentalis) are among the most significant invasive pests worldwide. In addition to causing direct plant damage, they transmit tomato spotted wilt virus (TSWV) (species Orthotospovirus tomatomaculae; genus Orthotospovirus), a member of the genus Orthotospovirus. Although numerous studies have examined virus-insect-host plant interactions, research on the TSWV-thrips-plant tripartite interaction remains limited. In this study, we found that F. occidentalis can induce plant defence responses. FoCSP1, a chemosensory protein from F. occidentalis, was identified as a salivary elicitor capable of inducing serial plant defence responses in Nicotiana benthamiana. Our results revealed that the FoCSP1-induced plant defence responses did not affect thrips feeding preference but significantly inhibited both offspring and egg production. Moreover, TSWV impairs these defence responses through its encoded proteins, N and NSs, thereby alleviating the FoCSP1-mediated suppression of thrips offspring and egg production. Collectively, these findings indicate that TSWV promotes the offspring and egg production of its thrips vector by inhibiting plant defences induced by FoCSP1, providing new insights into the TSWV-thrips-plant tripartite interaction.
Keywords: Frankliniella occidentalis; CSP1; plant defence responses; salivary elicitor; tomato spotted wilt virus.
© 2025 The Author(s). Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Abd‐El‐Haliem, A. M. , Hoogstrate S. W., and Schuurink R. C.. 2018. “A Robust Functional Genomics Approach to Identify Effector Genes Required for Thrips (<styled-content style="fixed-case"> Frankliniella occidentalis </styled-content>) Reproductive Performance on Tomato Leaf Discs.” Frontiers in Plant Science 9: 1852. - PMC - PubMed
-
- Abe, H. , Tomitaka Y., Shimoda T., et al. 2012. “Antagonistic Plant Defense System Regulated by Phytohormones Assists Interactions Among Vector Insect, Thrips and a Tospovirus.” Plant & Cell Physiology 53: 204–212. - PubMed
-
- Basu, S. , Varsani S., and Louis J.. 2018. “Altering Plant Defenses: Herbivore‐Associated Molecular Patterns and Effector Arsenal of Chewing Herbivores.” Molecular Plant–Microbe Interactions 31: 13–21. - PubMed
-
- Chen, J. , Zhao Y. X., Luo X. J., et al. 2023. “NLR Surveillance of Pathogen Interference With Hormone Receptors Induces Immunity.” Nature 614: e16. - PubMed
MeSH terms
Grants and funding
- 2023ZD04074/Biological Breeding-National Science and Technology Major Project
- 31972241/National Natural Science Foundation of China
- 32102169/National Natural Science Foundation of China
- 2022YFD1401200/National Key Research and Development Program of China
- BZ2023030/Jiangsu Key Technology R & D Program and International Science and Technology Cooperation Project
LinkOut - more resources
Full Text Sources

