Communication, Coordination, and Security for People With Multiple Sclerosis (COCOS-MS): A Randomized, Phase II Trial of Cross-sectoral Care and Case Management
- PMID: 40554666
- PMCID: PMC12599190
- DOI: 10.3238/arztebl.m2025.0097
Communication, Coordination, and Security for People With Multiple Sclerosis (COCOS-MS): A Randomized, Phase II Trial of Cross-sectoral Care and Case Management
Abstract
Background: People with severe multiple sclerosis (PsMS) have multidimensional, complex needs. COCOS-MS is an exploratory study of the effect of cross-sectoral care and case management (CCM) on these patients' quality of life, palliative care needs, and psychological distress, as well as caregivers' burden.
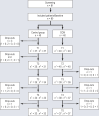
Methods: We conducted a randomized, controlled, phase II trial (DRKS00022771) with two parallel treatment arms: standard care versus CCM in addition to standard care over a 12-month period. A trial-specific CCM manual was used. The target variables were recorded in standardized fashion at baseline (T0) and every three months thereafter (T1 until the end of the intervention at T4, then T5 three months after the end of the intervention to determine sustainability). The primary endpoint was the change in the patients' quality of life (HALEMS) from baseline to month 12 in a group comparison. A modified intention-to-treat (mITT) was performed based on a mixed linear model with repeated measures over time (ARH1-structured covariance matrix). Values of p less than 0.05 were considered significant.
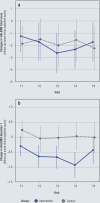
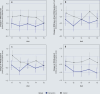
Results: 80 PsMS were randomly assigned 1:1 to one of the two treatment arms (male: female 1:2, median age 55 years [IQR 49-62], EDSS 6.5 [6-7.5], dropout rate 10%). There was no significant improvement in HALEMS after one year (T4) in the group comparison (-0.08 [-0.31, 0.15], p = 0.503; NNT = 22). Secondary endpoints such as subjective health status, psychological distress, and palliative care needs showed a clear response at the end of the intervention. After the intervention ended, the values approached the baseline level.
Conclusion: The primary endpoint did not reach significance in this health services research feasibility study. For further development of the study design, the focus will be on the psychological and palliative factors in which PsMS-a reference group for long-term neurological conditions-were found to benefit from the CCM.
Figures
References
-
- Deuschl G, Beghi E, Fazekas F, et al. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5:e551–e567. - PubMed
-
- Strupp J, Voltz R, Golla H. Opening locked doors: Integrating a palliative care approach into the management of patients with severe multiple sclerosis. Mult Scler. 2016;22:13–18. - PubMed
-
- Strupp J, Hartwig A, Golla H, Galushko M, Pfaff H, Voltz R. Feeling severely affected by multiple sclerosis: What does this mean? Palliat Med. 2012;26:1001–1010. - PubMed
-
- Kluger BM, Hudson P, Hanson LC, et al. Palliative care to support the needs of adults with neurological disease. Lancet Neurol. 2023;22:619–631. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

