Oscillating gradient spin echo diffusion time effects implicate variations in neurite beading for the heterogeneous reduced diffusion in human acute ischemic stroke lesions
- PMID: 40554725
- PMCID: PMC12393189
- DOI: 10.1002/mrm.30618
Oscillating gradient spin echo diffusion time effects implicate variations in neurite beading for the heterogeneous reduced diffusion in human acute ischemic stroke lesions
Abstract
Purpose: Monte Carlo simulations and short diffusion time measurements have suggested neurite beading and swelling as the underlying mechanism of reduced diffusion in acute stroke, although the observed diffusion time dependence is often heterogeneous and not yet fully understood. This study aimed to investigate the heterogeneity of diffusion time effects in ischemic lesions and explore the potential microstructural basis with Monte Carlo simulations.
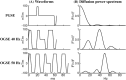
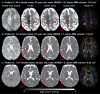
Methods: Pulsed gradient spin echo (PGSE, diffusion time 40 ms) and oscillating gradient spin echo (OGSE 40 Hz, diffusion time ˜5.1 ms) were acquired within 5 min in 39 acute ischemic stroke patients at 3 T. Mean, axial, and radial diffusivity differences between OGSE and PGSE (ΔMD/ΔAD/ΔRD) were compared between lesion and contralateral tissues (white and gray matter). Monte Carlo diffusion simulations of beaded axons for the experimental waveforms were used to investigate the effects of neurite morphology on time-dependent diffusivity changes.
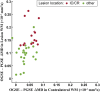
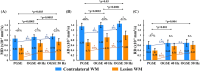
Results: PGSE yielded the typical mean diffusivity (MD) reduction of -40 ± 10% in ischemic lesions, whereas it was less at -29 ± 11% for OGSE 40 Hz. The OGSE-PGSE diffusion time difference was greater in lesions (ΔMD = 0.12 ± 0.06 × 10-3 mm2/s) than contralateral white matter (ΔMD = 0.04 ± 0.06 × 10-3 mm2/s), consistent with larger beading amplitude (0.18-0.43) and intracellular volume fraction (0.61-0.78) in lesions. ΔMD maps revealed regional variation with the largest effects in internal capsule and corona radiata.
Conclusion: This study found greater diffusion time effects in ischemic regions with purportedly larger axons. Monte Carlo simulations further support that the pronounced OGSE-PGSE diffusivity differences are expected in large axons with high beading amplitude.
Keywords: Monte Carlo diffusion simulation; neurite beading; oscillating gradient spin echo diffusion MRI; stroke.
© 2025 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures





Similar articles
-
Non-monotonic time- and frequency-dependent diffusion kurtosis in the human brain revealed by pulsed and oscillating gradient experiments.Magn Reson Med. 2025 Jul 17. doi: 10.1002/mrm.30635. Online ahead of print. Magn Reson Med. 2025. PMID: 40674625
-
Diffusion time dependency along the human corpus callosum and exploration of age and sex differences as assessed by oscillating gradient spin-echo diffusion tensor imaging.Neuroimage. 2020 Apr 15;210:116533. doi: 10.1016/j.neuroimage.2020.116533. Epub 2020 Jan 11. Neuroimage. 2020. PMID: 31935520
-
In vivo cortical microstructure mapping using high-gradient diffusion MRI accounting for intercompartmental water exchange effects.Neuroimage. 2025 Jul 1;314:121258. doi: 10.1016/j.neuroimage.2025.121258. Epub 2025 May 9. Neuroimage. 2025. PMID: 40349743 Free PMC article.
-
Engineering clinical translation of OGSE diffusion MRI.Magn Reson Med. 2025 Sep;94(3):913-936. doi: 10.1002/mrm.30510. Epub 2025 May 7. Magn Reson Med. 2025. PMID: 40331336 Free PMC article. Review.
-
White Matter Diffusion Abnormalities in Carotid Artery Disease: A Systematic Review and Meta-Analysis.J Neuroimaging. 2016 Sep;26(5):481-8. doi: 10.1111/jon.12347. Epub 2016 Apr 15. J Neuroimaging. 2016. PMID: 27079165
References
-
- Moseley ME, Cohen Y, Mintorovitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion‐and T2‐weighted MRI and spectroscopy. Magn Reson Med. 1990;14:330‐346. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

