Innate visual attraction before, during and after escape from adverse substrates in carpenter ants
- PMID: 40554759
- PMCID: PMC12276813
- DOI: 10.1242/jeb.250278
Innate visual attraction before, during and after escape from adverse substrates in carpenter ants
Abstract
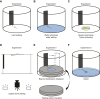
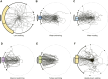
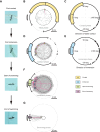
Many animals exhibit an innate attraction to dark areas or objects, driving orientation behaviours such as beacon aiming. In ants, some species do not appear to display beacon aiming. Here, we show that in one such species, Camponotus japonicus, the behaviour is triggered when crossing liquid-covered surfaces, regardless of locomotor pattern and the presence of water in the liquid. Once initiated, beacon aiming persisted even after the ants transitioned from water to dry substrates, as evidenced by their reorientation towards a displaced beacon. Beacon aiming could be observed before the ants fully transitioned from a dry substrate to a liquid-covered surface: when the ants were isolated on a water-surrounded platform, attraction to a beacon emerged while they were contacting the water, before finally deciding to swim towards the beacon. Adverse substrate conditions in general appear to be a factor triggering beacon aiming as we also identified one condition (so far) in which even liquid immersion was not required for beacon aiming, namely upside-down walking. These results indicate that beacon aiming in C. japonicus is performed before, during and after escape from adverse substrates. Evidence that substrate conditions can alter seemingly hardwired responses suggests that insects may adjust even simple behaviours in response to environmental conditions in a more sensitive way than commonly assumed.
Keywords: Innate behaviour; Landmark; Substrate conditions; Swimming; Visual orientation; Walking.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Adis, J. (1982). Eco-entomological observations from the Amazon: III. How do leafcutting ants of inundation forests survive flooding? Acta Amazon 12, 839-840. 10.1590/1809-43921982124839 - DOI
-
- Akiba, M., Sugimoto, K., Aoki, R., Murakami, R., Miyashita, T., Hashimoto, R., Hiranuma, A., Yamauchi, J., Ueno, T. and Morimoto, T. (2020). Dopamine modulates the optomotor response to unreliable visual stimuli in Drosophila melanogaster. Eur. J. Neurosci. 51, 822-839. 10.1111/ejn.14648 - DOI - PubMed
-
- Avellanal, M. H., Jaramillo, E., Naylor, E. and Kennedy, F. (2000). Orientation of Phalerisida maculata Kulzer (Coleoptera, Tenebrionidae) in sandy beaches of the Chilean coast: orientation of Phalerisida maculata in sandy beaches. J. Exp. Mar. Biol. Ecol. 247, 153-167. 10.1016/S0022-0981(99)00185-9 - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

