Regulatory mechanisms of the Hippo/YAP axis by G-protein coupled estrogen receptor in gastric signet-ring cell carcinoma
- PMID: 40554953
- PMCID: PMC12226116
- DOI: 10.1016/j.neo.2025.101199
Regulatory mechanisms of the Hippo/YAP axis by G-protein coupled estrogen receptor in gastric signet-ring cell carcinoma
Abstract
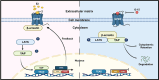
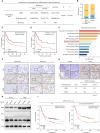
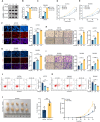
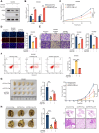
Although aberrant activation of the Hippo/YAP axis has been implicated in the development of gastric cancer, functional studies of this cascade in the context of gastric signet-ring cell carcinoma (GSRC) remain absent. Our previous single-cell sequencing results showed that G protein-coupled estrogen receptor (GPER) is overexpressed in GSRC, and this overexpression is associated with aberrant activation of the Hippo/YAP axis. In this study, we integrated in vitro cytological functional assays with GSRC cell lines and in vivo xenograft nude mice models to elucidate the functional implications of GPER in GSRC. The overexpression of GPER was identified as being associated with more unfavorable outcomes in GSRC. Its activation facilitated tumor proliferation by YAP nuclear translocation and subsequent transcriptional activation. Mechanistically, GPER inhibited LATS1-mediated YAP phosphorylation by competitively binding to ARRB2, thereby enhancing YAP activity. Moreover, YAP was shown to bind to the GPER promoter, forming a positive feedback loop that reinforced oncogenic signaling. Pharmacological inhibition of GPER using G-15 reduced YAP activation and effectively attenuated tumor aggressiveness, highlighting the GPER-YAP feedback loop as a potential therapeutic target for GSRC. This study underscores the pivotal role of the GPER-YAP positive feedback loop in GSRC and proposes dual inhibition of GPER and YAP as a promising therapeutic strategy for GSRC.
Keywords: Carcinogenesis; G protein-coupled estrogen receptor; Gastric signet-ring cell carcinoma; Hippo/YAP axis; Positive feedback loop.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jie Chai reports financial support was provided by National Natural Science Foundation of China. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
The interplay between oxytocin receptor and YAP in regulating gastric cancer progression.Oncogene. 2025 Sep;44(34):3126-3141. doi: 10.1038/s41388-025-03480-3. Epub 2025 Jul 2. Oncogene. 2025. PMID: 40604321
-
STK3 is a transcriptional target of YAP1 and a hub component in the crosstalk between Hippo and Wnt signaling pathways during gastric carcinogenesis.Mol Cancer. 2025 Jul 2;24(1):186. doi: 10.1186/s12943-025-02391-x. Mol Cancer. 2025. PMID: 40604818 Free PMC article.
-
YAP as a therapeutic target to reverse trastuzumab resistance.Gastric Cancer. 2025 Sep;28(5):799-813. doi: 10.1007/s10120-025-01630-w. Epub 2025 Jun 20. Gastric Cancer. 2025. PMID: 40542295 Free PMC article.
-
YAP as a therapeutic target in esophageal squamous cell carcinoma: insights and strategies.Ann Med. 2025 Dec;57(1):2536200. doi: 10.1080/07853890.2025.2536200. Epub 2025 Jul 22. Ann Med. 2025. PMID: 40693409 Free PMC article. Review.
-
Exploring Hippo YAP/TAZ Signaling: A Novel Avenue for Cardiovascular Disorders.Cell Biol Int. 2025 Sep;49(9):1079-1101. doi: 10.1002/cbin.70052. Epub 2025 Jul 14. Cell Biol Int. 2025. PMID: 40654305 Review.
References
-
- Mariette C., Carneiro F., Grabsch H.I., van der Post R.S., Allum W., de Manzoni G. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1–9. - PubMed
-
- Kwon K.J., Shim K.N., Song E.M., Choi J.Y., Kim S.E., Jung H.K., et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17:43–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

