Screening and identification of HLA-A2-restricted neoepitopes for immunotherapy in endocrine therapy-resistant breast cancer
- PMID: 40554954
- PMCID: PMC12240116
- DOI: 10.1016/j.neo.2025.101200
Screening and identification of HLA-A2-restricted neoepitopes for immunotherapy in endocrine therapy-resistant breast cancer
Abstract
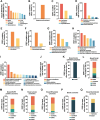
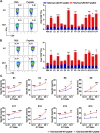
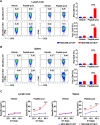
Endocrine therapy has shown significant clinical efficacy in estrogen receptor alpha (ERα)-positive breast cancer management, but the emergence of therapy-resistant mutations significantly undermines treatment outcomes, frequently leading to disease progression and metastasis. Among these resistance mechanisms, mutations in the ESR1 gene are particularly prevalent, detectable in 76% of endocrine therapy-resistant tumor specimens. The identification of immunogenic neoepitopes derived from mutant ESR1 offers a promising therapeutic avenue for patients with endocrine therapy-resistant breast cancer. In this study, we systematically investigated the mutational landscape of ESR1 across various cancer types, with particular emphasis on mutation frequency and spectrum analysis. Our findings revealed that non-synonymous ESR1 mutations predominantly occurred in breast cancer, clustering at four distinct hotspot sites: K303, E380, Y537 and D538. We further characterized the mutation prevalence at these hotspots across different breast cancer subtypes. Through comprehensive screening, we identified eight human leukocyte antigen (HLA)-A*0201 restricted immunogenic neoepitopes derived from ESR1 hotspot mutations. These neoepitopes demonstrated the capacity to elicit specific cytotoxic T lymphocytes (CTLs) responses both in vitro and in vivo. The induced CTLs exhibited specific recognition and cytotoxic activity against both T2A2 cells loaded with mutant neoepitopes and HLA-A*0201-positive breast cancer cells transfected with minigene encoding mutant neoepitopes. Notably, adoptive transfer of T cells primed with a peptide pool containing these eight neoepitopes significantly suppressed tumor growth and enhanced CD8+ T cells infiltration within tumor tissue. These findings suggest that the identified neoepitopes represent promising candidates for the development of tumor shared neoantigen vaccines.
Keywords: ESR1; HLA-A2; Hotspot mutations; Immunotherapy; Neoepitopes.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Integrative Computational Immunology Applied to Identify and Characterize CD8+ T-cell Neoepitopes of Shared KRAS Neoantigen Oncogenic Driver Hotspot Mutations.Asian Pac J Cancer Prev. 2025 Jul 1;26(7):2413-2423. doi: 10.31557/APJCP.2025.26.7.2413. Asian Pac J Cancer Prev. 2025. PMID: 40729063
-
Identification of 68 HLA-A24 and -A2-restricted cytotoxic T lymphocyte-inducing peptides derived from 10 common cancer-specific antigens frequently expressed in various solid cancers.Neoplasia. 2025 Mar;61:101135. doi: 10.1016/j.neo.2025.101135. Epub 2025 Feb 11. Neoplasia. 2025. PMID: 39938154 Free PMC article.
-
PARP-1 as a novel target in endocrine-resistant breast cancer.J Exp Clin Cancer Res. 2025 Jun 16;44(1):175. doi: 10.1186/s13046-025-03441-4. J Exp Clin Cancer Res. 2025. PMID: 40518539 Free PMC article.
-
The Efficacy of Neoantigen-Loaded Dendritic Cell Vaccine Immunotherapy in Non-Metastatic Gastric Cancer.Med Sci (Basel). 2025 Jul 11;13(3):90. doi: 10.3390/medsci13030090. Med Sci (Basel). 2025. PMID: 40700119 Free PMC article. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Balamurugan K., Poria D.K., Sehareen S.W., Krishnamurthy S., Tang W., McKennett L., Padmanaban V., Czarra K., Ewald A.J., Ueno N.T., Ambs S., Sharan S., Sterneck E. Stabilization of E-cadherin adhesions by COX-2/GSK3β signaling is a targetable pathway in metastatic breast cancer. JCI. Insight. 2023;8 - PMC - PubMed
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Rugo H.S., Diéras V., Gelmon K.A., Finn R.S., Slamon D.J., Martin M., Neven P., Shparyk Y., Mori A., Lu D.R., Bhattacharyya H., Bartlett C., Iyer S., Johnston S., Ettl J., Harbeck N. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann. Oncol. 2018;29:888–894. - PMC - PubMed
-
- Patel H.K., Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018;186:1–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

