ALS Mutations Shift the Isoelectric Point of the KIF5A C Terminal Inducing Protein Aggregation and TDP-43 Mislocalization
- PMID: 40555518
- PMCID: PMC12311770
- DOI: 10.1523/JNEUROSCI.1658-24.2025
ALS Mutations Shift the Isoelectric Point of the KIF5A C Terminal Inducing Protein Aggregation and TDP-43 Mislocalization
Abstract
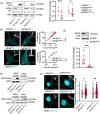
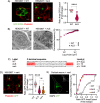
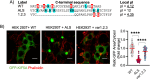
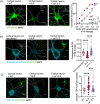
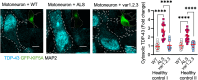
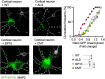
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by death of lower and upper motor neurons. Although the mechanism behind the selective neuron loss is still unclear, several heterogeneous genes have been causally linked to ALS. KIF5A encodes for a neuronally enriched kinesin involved in protein transport, and mutations within this gene have been causally linked to different motor neuron diseases. The mutations identified in ALS patients are mostly predicted to alter its mRNA splicing, leading to a frameshift mutation and an aberrant 39-aa-long sequence in the C-terminal domain of KIF5A. Here we found that ALS-related KIF5A mutations induce the accumulation of the mutant form of the protein in human motoneurons, which are also characterized by the cytosolic mislocalization of TDP-43. This ALS hallmark was even exacerbated upon overexpression of the ALS-KIF5A protein in cells differentiated from healthy controls and primary neurons, suggesting a pathological connection between the cellular load of the mutant protein and TDP-43 pathology. While the terminal domain of the WT isoform is characterized by an acid isoelectric point (pI), the ALS variant presents a basic pI due to the altered aminoacidic composition of this sequence. We thus generated a KIF5A-ALS isoform that retained part of the aberrant sequence but with lower pI. The overexpression of this mutated variant led to significantly lower protein aggregation and TDP-43 mislocalization than the ALS mutant. Our data show that re-establishing the correct pI rescues KIFA aggregation and significantly reduces the cytoplasmic mislocalization of TDP-43.
Keywords: ALS; KIF5A; TDP-43; aggregation; isoelectric point.
Copyright © 2025 Zanella et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
