Apolipoprotein M attenuates age-related macular degeneration phenotypes via sphingosine-1-phosphate signaling and lysosomal lipid catabolism
- PMID: 40555726
- PMCID: PMC12187922
- DOI: 10.1038/s41467-025-60830-1
Apolipoprotein M attenuates age-related macular degeneration phenotypes via sphingosine-1-phosphate signaling and lysosomal lipid catabolism
Abstract
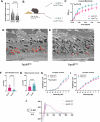
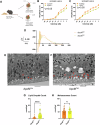
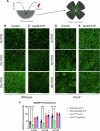
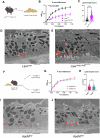
Age-related macular degeneration (AMD) is a leading cause of blindness in people over 50. AMD and cardiovascular disease share risk factors including age, impaired lipid metabolism, and extracellular lipid deposition. Because of its importance in age-related diseases, we hypothesize that apolipoprotein M (ApoM), a lipocalin that binds sphingosine-1-phosphate (S1P), might restore lipid homeostasis and retinal function in AMD. In support, we find that human patients with AMD demonstrate significantly reduced ApoM compared to controls. In mice with impaired retinal cholesterol efflux, ApoM improves retinal pigment epithelium (RPE) function and lipotoxicity in an S1P- and S1P receptor 3-dependent manner. Ultrastructural evidence of enhanced melanosome-lipid droplet interactions led us to hypothesize and demonstrate that ApoM-S1P signaling drives RPE-specific lysosomal lipid catabolism. RPE-specific knockout of lysosomal acid lipase recapitulates features of AMD. Our study defines a novel role for ApoM/S1P signaling in AMD driven by RPE lipotoxicity, mediated by cell-autonomous lysosomal lipid catabolism.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: RSA and AJ have intellectual property applications licensed by Washington University to Mobius Scientific. RSA and AJ are on the advisory board of Mobius Scientific, and RSA is currently the CSO at Mobius Scientific. The remaining authors declare no competing interests.
Figures



References
-
- Wong, W. L. et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health2, e106–e116 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

