Tirzepatide on ingestive behavior in adults with overweight or obesity: a randomized 6-week phase 1 trial
- PMID: 40555748
- PMCID: PMC12443625
- DOI: 10.1038/s41591-025-03774-9
Tirzepatide on ingestive behavior in adults with overweight or obesity: a randomized 6-week phase 1 trial
Abstract
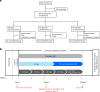
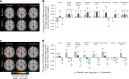
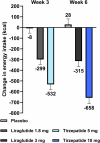
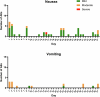
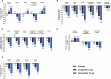
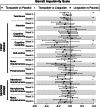
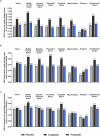
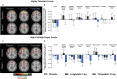
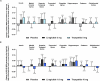
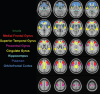
Tirzepatide induces weight reduction but the underlying mechanisms are unknown. This 6-week phase 1 study investigated early effects of tirzepatide on energy intake. Male and female adults without diabetes (n = 114) and a body mass index from 27 to 50 kg per m2 were randomized 1:1:1 to blinded once-weekly tirzepatide or placebo, or open-label once-daily liraglutide. The primary outcome was change from baseline to week 3 in energy intake during an ad libitum lunch with tirzepatide versus placebo. Secondary outcomes assessed self-reported ingestive behavior and blood-oxygenation-level-dependent functional magnetic resonance imaging with food photos. Tirzepatide reduced energy intake versus placebo at week 3 (estimated treatment difference -524.6 kcal (95% confidence interval -648.1 to -401.0), P < 0.0001). With regard to secondary outcomes versus placebo, tirzepatide decreased overall appetite, food cravings, tendency to overeat, perceived hunger and reactivity to foods in the environment but did not impact volitional restriction of dietary intake. At week 3 versus placebo, tirzepatide did not statistically significantly impact blood-oxygenation-level-dependent activation to highly palatable food photos (aggregated category of high-fat, high-sugar foods and high-fat, high-carbohydrate foods) but decreased activation to high-fat, high-sugar food photos in the medial frontal and cingulate gyri, orbitofrontal cortex and hippocampus. Our results suggest tirzepatide reduces food intake, potentially by impacting ingestive behavior. ClinicalTrials.gov registration: NCT04311411 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The institution of C.K.M. is supported by NORC Center Grant P30 DK072476 entitled Nutrition and Metabolic Health Through the Lifespan sponsored by NIDDK and by grant U54 GM104940 from the National Institute of General Medical Sciences, which funds the Louisiana Clinical and Translational Science Center. C.K.M. declares research funding from Eli Lilly and Company paid to his institution to perform the work described in this paper; research grants or contracts paid to his institution from Pack Health, American Society for Nutrition, RAND Corporation, Richard King Mellon Foundation (RKMF), Evidation Health, Leona M. and Harry B. Helmsley Charitable Trust, State of Louisiana Federal American Rescue Plan (ARP), USDA, The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., University of Rochester (NY), Foundation for Food and Agriculture Research, Kroger Co. Zero Hunger/Zero Waste Foundation, National Institute for Health Research (NIHR), Weight Watchers, American Diabetes Association, Eli Lilly and Company, National Science Foundation (NSF) and National Institutes of Health (NIH); royalties from ABGIL; personal consulting fees from EHE Health and WondrHealth; honoraria from Obesity Action Coalition, Indiana University Bloomington, University of Alabama at Birmingham, Nutrition Obesity Research Center, Brigham Young University, University of Kansas Medical Center (KUMC), University of Southern California and Commission on Dietetic Registration; travel support from Indiana University Bloomington, University of Alabama at Birmingham, Nutrition Obesity Research Center, Brigham Young University, University of Kansas Medical Center (KUMC), University of Southern California and Commission on Dietetic Registration; provision of research materials from Eli Lilly and Company and Weight Watchers; participation on advisory boards for Duke University (NIH-funded trial) and University of Alabama at Birmingham NIH-funded Nutrition Obesity Research Center; mentorship for a junior investigator at the University of Nebraska–Lincoln who received an NIH-supported training grant; and participation in a Bray Course Planning Committee. O.T.C. declares a research grant from Eli Lilly and Company to his institution to perform the work described in this paper and research grants from NIH and Nestle, Inc., and has served on advisory boards for Novo Nordisk. D.A.K. declares clinical trial and research support from Eli Lilly and Company to his institution to perform the work described in this paper, as well as research support from the NIH. R.V.C. declares clinical trial support from Eli Lilly and Company and research support from Adipo Therapeutics. R.D.M. declares research grants from Grain Foods Foundation, Almond Board of California and Eli Lilly and Company; consulting fees from Mars Foods, General Mills Bright Seed and the Calorie Control Council; honoraria from Clean Label Conference, Columbia University, Sports Nutrition Plus, USDA, Michigan State University, American Diabetes Association, Mediterranean Diet Roundtable, American Society for Nutrition, American Italian Food Coalition and Healthy Aging Science Forum; participation on safety monitoring boards/advisory boards for NIDCD; and presidency of the American Society for Nutrition. U.D. declares a research grant from Eli Lilly and Company to her institution to perform the work described in this paper and research support from the NIH and the International Manganese Institute. S.C. declares payments to her institution to support the work described in this paper. S.S., H.N., A.K., S.U., L.B., E.P., A.H., M.M., D.O.S., Z.M. and T.C. are employees and shareholders of Eli Lilly and Company. D.S. declares no competing interests.
Figures



References
-
- Bray, G. A., Kim, K. K., Wilding, J. P. H. & World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev.18, 715–723 (2017). - PubMed
-
- Obesity and Overweight Key Facts. 2021 (World Health Organization, accessed 16 February 2024); www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
-
- Chetty, A. K., Rafi, E., Bellini, N. J., Buchholz, N. & Isaacs, D. A review of incretin therapies approved and in late-stage development for overweight and obesity management. Endocr. Pract.30, 292–303 (2024). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

