The impact of inoculation with the inactivated COVID-19 vaccine on the outcomes of in vitro fertilization and embryo transfer: a cohort study of 1,258 women from Sichuan, China
- PMID: 40556827
- PMCID: PMC12185302
- DOI: 10.3389/fendo.2025.1491259
The impact of inoculation with the inactivated COVID-19 vaccine on the outcomes of in vitro fertilization and embryo transfer: a cohort study of 1,258 women from Sichuan, China
Abstract
Objective: This study aimed to assess the impact of inoculation with the inactivated coronavirus disease 2019 (COVID-19) vaccine on the outcomes of in vitro fertilization and embryo transfer (IVF-ET).
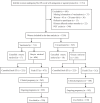
Methods: From January 2021 to December 2022, patients undergoing their first cycle of IVF-ET at the Reproductive Medicine Center of Sichuan Provincial Women's and Children's Hospital were prospectively enrolled. Based on inoculation with inactivated COVID-19 vaccines before ovarian stimulation (OS) by a gonadotrophin-releasing hormone (GnRH) antagonist or agonist protocol, the patients were divided into the vaccinated group (n = 713) and the unvaccinated group (n = 545). The vaccinated group were sub-grouped based on the dose of inoculation (single dose, n = 74; double dose, n = 275; and triple dose, n = 126) and the interval between the first inoculation and OS (<3 months, n = 65; 3-6 months, n = 123; and >6 months, n = 287).
Results: The rates of mature oocytes, normal fertilization, cleavage embryo, high-quality cleavage embryo, blastocysts, and high-quality blastocysts were not significantly different between the vaccinated and unvaccinated groups (p > 0.05). For fresh embryo transfer, the implantation rate (IR), the clinical pregnancy rate (CPR), the live birth rate (LBR), the gestational age at delivery, and the birth weight of infants were not significantly different between the two groups (p > 0.05). The IR, CPR, LBR, and birth weight of infants were not significantly different for both the dose and interval subgroups (p > 0.05).
Conclusion: Inactivated COVID-19 vaccines may not affect the outcomes of IVF-ET.
Keywords: SARS-CoV-2; embryo transfer; in vitro fertilization; inactivated vaccine; live birth.
Copyright © 2025 Wei, Qiu, Leng, Chen, Liang, Deng, Ma, Hei, Li-Ling and Gong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Associations between inactivated COVID-19 vaccination status and timing and fertility and pregnancy outcomes following frozen-thawed embryo transfer: a prospective cohort study.Front Endocrinol (Lausanne). 2025 Jun 26;16:1587251. doi: 10.3389/fendo.2025.1587251. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40642511 Free PMC article.
-
Oral medications including clomiphene citrate or aromatase inhibitors with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation.Cochrane Database Syst Rev. 2017 Nov 2;11(11):CD008528. doi: 10.1002/14651858.CD008528.pub3. Cochrane Database Syst Rev. 2017. PMID: 29096046 Free PMC article.
-
Cycle regimens for frozen-thawed embryo transfer.Cochrane Database Syst Rev. 2017 Jul 5;7(7):CD003414. doi: 10.1002/14651858.CD003414.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2025 Jun 3;6:CD003414. doi: 10.1002/14651858.CD003414.pub4. PMID: 28675921 Free PMC article. Updated.
-
Shorter ovarian stimulation is detrimental to fresh embryo transfer outcomes in PCOS women undergoing GnRH antagonist protocol: a retrospective cohort study.Sci Rep. 2025 Jul 1;15(1):21002. doi: 10.1038/s41598-025-06217-0. Sci Rep. 2025. PMID: 40594896 Free PMC article.
-
Association between inactivated COVID-19 vaccine and semen quality among males recovered from omicron infection: a retrospective cohort study.Expert Rev Clin Immunol. 2025 Jun;21(6):825-834. doi: 10.1080/1744666X.2025.2507329. Epub 2025 May 26. Expert Rev Clin Immunol. 2025. PMID: 40372240
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous