Longitudinal associations between amyloid and symptoms of depression and anxiety in subjective cognitive decline: the impact of personality characteristics
- PMID: 40557139
- PMCID: PMC12185448
- DOI: 10.3389/fpsyt.2025.1572174
Longitudinal associations between amyloid and symptoms of depression and anxiety in subjective cognitive decline: the impact of personality characteristics
Abstract
Introduction: Depressive/anxiety symptoms are common in subjective cognitive decline (SCD) and may relate to Alzheimer's pathology, potentially modulated by personality characteristics.
Methods: Depressive/anxiety symptoms were assessed over 4 ± 2 years in 329 SCD (88 amyloid-positive/241 amyloid-negative) using Geriatric Depression Scale-15 (GDS), Center for Epidemiological Studies-Depression (CES-D), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Mixed-effects models assessed associations between amyloid status and these symptoms, with neuroticism and somatization as effect-modifiers.
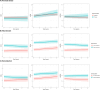
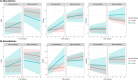
Results: Amyloid status was not directly associated with GDS, CES-D or HADS-A. However, neuroticism modified the association between amyloid status and GDS (p<0.05). In lower neuroticism, amyloid positivity was associated with GDS increase (β:0.10 ± 0.08), but not in higher neuroticism (β:-0.04 ± 0.12). Somatization modified the association between amyloid status and CES-D (p<0.05). In lower somatization, amyloid positivity was associated with CES-D increase (β:0.65 ± 0.23), but not in higher somatization (β:-0.12 ± 0.29).
Discussion: Amyloid-positive individuals with lower neuroticism/somatization increased more in depressive symptoms over time, suggesting a preclinical AD-related depressive phenotype.
Keywords: Alzheimer’s disease; anxiety; depression; neuroticism; somatization; subjective cognitive decline.
Copyright © 2025 Trieu, van Leeuwenstijn, Schlüter, Ebenau, Verberk, Sikkes, Verfaillie, van de Giessen, Teunissen, van der Flier and van Harten.
Conflict of interest statement
WV reports that her research programs have been funded by ZonMW, NWO, EU-JPND, EU-IHI, Alzheimer Nederland, Hersenstichting, CardioVascular Onderzoek Nederland, HealthHolland, Topsector Life Sciences & Health, Stichting Dioraphte, Gieskes-Strijbis Fonds, Stichting Equilibrio, Edwin Bouw Fonds, Pasman Stichting, Stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc., Novartis-NL, Life-MI, AVID, Roche BV, Eli Lilly-NL, Fujifilm, Eisai, and Combinostics. WV holds the Pasman Chair. WV is the recipient of ABOARD, a public-private partnership funded by ZonMW #73305095007 and HealthHolland, Topsector Life Sciences & Health PPP-allowance; #LSHM20106. WV is also the recipient of TAP-dementia, funded by ZonMW #10510032120003, with co-financing from Avid Radiopharmaceuticals and Amprion. All funding is paid to her institution. WV has been an invited speaker at Biogen MA Inc., Danone, Eisai, WebMD Neurology Medscape, Novo Nordisk, Springer Healthcare, and the European Brain Council, with all funding paid to her institution. WV has consulted for Oxford Health Policy Forum CIC, Roche, Biogen MA Inc., and Eisai, with all funding paid to her institution. WV has participated in advisory boards for Biogen MA Inc., Roche, and Eli Lilly and is a member of the steering committee of EVOKE/EVOKE+ Novo Nordisk. She is also a member of the steering committee of PAVE and Think Brain Health. WV was associate editor of Alzheimer Research & Therapy in 2020/2021 and is currently an associate editor at Brain. CET reports that her research is supported by the European Commission through several grants, including the Marie Curie International Training Network grant agreement No. 860197, MIRIADE, TAME, Innovative Medicines Initiatives 3TR Horizon 2020, grant No. 831434, EPND IMI 2 Joint Undertaking JU, grant No. 101034344, JPND bPRIDE, CCAD, and the European Partnership on Metrology, co-financed by the European Union’s Horizon Europe Research and Innovation Programme and Participating States 22HLT07 NEuroBioStand. CET is also supported by the CANTATE project funded by the Alzheimer Drug Discovery Foundation, the Alzheimer Association, the Michael J. Fox Foundation, Health Holland, the Dutch Research Council ZonMW, the Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, and Alzheimer Netherlands. CET is the recipient of ABOARD, a public-private partnership funded by ZonMW #73305095007 and Health~Holland, Topsector Life Sciences & Health PPP-allowance; #LSHM20106, and TAP-dementia, a project funded by ZonMW #10510032120003 in the context of the Dutch National Dementia Strategy. CET has research contracts with Acumen, ADx Neurosciences, AC-Immune, Alamar, Aribio, Axon Neurosciences, Beckman-Coulter, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Eli Lilly, Fujirebio, Instant Nano Biosensors, Novo Nordisk, Olink, PeopleBio, Quanterix, Roche, Toyama, and Vivoryon. CET is the editor-in-chief of Alzheimer Research & Therapy and serves on the editorial boards of Molecular Neurodegeneration, Neurology: Neuroimmunology & Neuroinflammation, and Medidact Neurologie/Springer. CET also serves on committees defining guidelines for cognitive disturbances and acute neurology in the Netherlands. CET has had consultancy and speaker contracts with Aribio, Biogen, Beckman-Coulter, Cognition Therapeutics, Eli Lilly, Merck, Novo Nordisk, Olink, Roche, and Veravas. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

