Protocol: the International Milk Composition (IMiC) Consortium - a harmonized secondary analysis of human milk from four studies
- PMID: 40557242
- PMCID: PMC12186657
- DOI: 10.3389/fnut.2025.1548739
Protocol: the International Milk Composition (IMiC) Consortium - a harmonized secondary analysis of human milk from four studies
Abstract
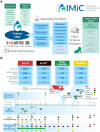
Introduction: Human milk (HM) contains a multitude of nutritive and nonnutritive bioactive compounds that support infant growth, immunity and development, yet its complex composition remains poorly understood. Integrating diverse scientific disciplines from nutrition and global health to data science, the International Milk Composition (IMiC) Consortium was established to undertake a comprehensive harmonized analysis of HM from low, middle and high-resource settings to inform novel strategies for supporting maternal-child nutrition and health.
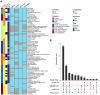
Methods and analysis: IMiC is a collaboration of HM experts, data scientists and four mother-infant health studies, each contributing a subset of participants: Canada (CHILD Cohort, n = 400), Tanzania (ELICIT Trial, n = 200), Pakistan (VITAL-LW Trial, n = 150), and Burkina Faso (MISAME-3 Trial, n = 290). Altogether IMiC includes 1,946 HM samples across time-points ranging from birth to 5 months. Using HM-validated assays, we are measuring macronutrients, minerals, B-vitamins, fat-soluble vitamins, HM oligosaccharides, selected bioactive proteins, and untargeted metabolites, proteins, and bacteria. Multi-modal machine learning methods (extreme gradient boosting with late fusion and two-layered cross-validation) will be applied to predict infant growth and identify determinants of HM variation. Feature selection and pathway enrichment analyses will identify key HM components and biological pathways, respectively. While participant data (e.g., maternal characteristics, health, household characteristics) will be harmonized across studies to the extent possible, we will also employ a meta-analytic structure approach where HM effects will be estimated separately within each study, and then meta-analyzed across studies.
Ethics and dissemination: IMiC was approved by the human research ethics board at the University of Manitoba. Contributing studies were approved by their respective primary institutions and local study centers, with all participants providing informed consent. Aiming to inform maternal, newborn, and infant nutritional recommendations and interventions, results will be disseminated through Open Access platforms, and data will be available for secondary analysis.
Clinical trial registration: ClinicalTrials.gov, identifier, NCT05119166.
Keywords: breastfeeding; human milk; infant growth; infant nutrition; machine learning.
Copyright © 2025 Fehr, Mertens, Shu, Dailey-Chwalibóg, Shenhav, Allen, Beggs, Bode, Chooniedass, DeBoer, Deng, Espinosa, Hampel, Jahual, Jehan, Jain, Kolsteren, Kawle, Lagerborg, Manus, Mataraso, McDermid, Muhammad, Peymani, Pham, Shahab-Ferdows, Shafiq, Subramoney, Sunko, Toe, Turvey, Xue, Rodriguez, Hubbard, Aghaeepour and Azad.
Conflict of interest statement
VS was employed by DVPL Tech. MA has received speaking honoraria from non-profit organizations that support breastfeeding (Institute for the Advancement of Breastfeeding & Lactation Education, Thai Breastfeeding Centre, UK Baby Friendly, Kansas Breastfeeding Coalition), and companies that produce human milk-related products (Prolacta Biosciences, Medela). She is a scientific advisor to TinyHealth (an infant microbiome testing company) and has consulted for DSM (an HMO manufacturer). MJ and KL hold equity and a position at Sapient Bioanalytics, LLC. PKa was employed by Cytel. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- WHO, UNICEF . Global strategy for infant and young child feeding. World Health Organization; Geneva, Switzerland. (2003). Available online at: https://www.who.int/publications/i/item/9241562218 (Accessed October 3, 2024).
Associated data
LinkOut - more resources
Full Text Sources
Medical

