Oncogenic viruses rewire the epigenome in human cancer
- PMID: 40557320
- PMCID: PMC12185521
- DOI: 10.3389/fcimb.2025.1617198
Oncogenic viruses rewire the epigenome in human cancer
Abstract
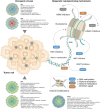
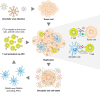
Viruses contribute to approximately 15-20% of global cancer cases, yet the full spectrum of their oncogenic mechanisms continues to be uncovered. Beyond the classical roles of genome integration, chronic inflammation, and immune evasion, mounting evidence reveals that oncogenic viruses-including the human papillomavirus (HPV), Epstein-Barr virus (EBV), hepatitis B virus (HBV), hepatitis C virus (HCV), and Human T-cell leukemia virus type 1 (HTLV-1)-profoundly reshape the host epigenome to establish persistent infection and promote tumorigenesis. These viruses orchestrate widespread and durable changes in DNA methylation, histone modification, chromatin accessibility, and non-coding RNA expression, silencing tumor suppressors, deregulating oncogenic pathways, and inducing stemness-like phenotypes. In this review, we provide a comprehensive synthesis of how distinct oncogenic viruses modulate the epigenetic landscape across tissue contexts, with a focus on cervical, hepatic, and lymphoepithelial cancers. We also explore how these virus-induced epigenetic "scars" may persist after viral clearance and highlight recent advances in therapeutic targeting. Emerging therapeutic strategies that integrate oncolytic virotherapy, epigenetic drugs, and immune modulation through combinational therapy offer synergistic mechanisms to overcome immune resistance and epigenetic silencing in virus-induced cancers. These integrated approaches hold transformative potential for more durable and targeted treatment outcomes.
Keywords: cancer; drugs; epigenome; immune modulation; oncogenic virus; oncolytic virotherapy.
Copyright © 2025 Bautista and Lopez-Cortes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The impact of DNA tumor viruses in low-to-middle income countries (LMICS): A literature review.Tumour Virus Res. 2024 Dec;18:200289. doi: 10.1016/j.tvr.2024.200289. Epub 2024 Jul 6. Tumour Virus Res. 2024. PMID: 38977263 Free PMC article. Review.
-
[Epigenetics' implication in autism spectrum disorders: A review].Encephale. 2017 Aug;43(4):374-381. doi: 10.1016/j.encep.2016.07.007. Epub 2016 Sep 28. Encephale. 2017. PMID: 27692350 French.
-
A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection.J Virol. 2017 Mar 29;91(8):e02388-16. doi: 10.1128/JVI.02388-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148804 Free PMC article.
-
Epigenetic editing and epi-drugs: a combination strategy to simultaneously target KDM4 as a novel anticancer approach.Clin Epigenetics. 2025 Jun 19;17(1):105. doi: 10.1186/s13148-025-01913-0. Clin Epigenetics. 2025. PMID: 40537846 Free PMC article.
-
The role of pioneering transcription factors, chromatin accessibility and epigenetic reprogramming in oncogenic viruses.Front Microbiol. 2025 Jun 16;16:1602497. doi: 10.3389/fmicb.2025.1602497. eCollection 2025. Front Microbiol. 2025. PMID: 40589575 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

