Fungi and cancer: unveiling the complex role of fungal infections in tumor biology and therapeutic resistance
- PMID: 40557321
- PMCID: PMC12185418
- DOI: 10.3389/fcimb.2025.1596688
Fungi and cancer: unveiling the complex role of fungal infections in tumor biology and therapeutic resistance
Abstract
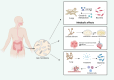
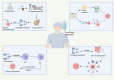
Cancer remains one of the most significant causes of mortality across the world. Despite remarkable advancements made in early detection, therapeutic strategies, and the advent of immunotherapy in recent years, numerous challenges continue to hinder optimal outcomes. The development and progression of cancer are driven not only by genetic and epigenetic alterations within tumor cells but also by dynamic interactions occurring with the surrounding tumor microenvironment (TME). It is a highly complex milieu composed of tumor cells, non-tumor stromal cells, extracellular matrix components, immune cells, blood vessels, and diverse signaling molecules. Emerging evidence underscores the pivotal role of fungi in influencing cancer biology, including initiation, progression, immune evasion, and the modulation of TME. Fungi, which are omnipresent microorganisms, have traditionally been considered opportunistic pathogens. However, recent research highlights their broader impact on host immunity and their potential contributions to cancer pathogenesis. For instance, in patients with cancer, fungal infections not only exacerbate clinical complications but also create conditions conducive to tumor growth, metastasis, and immune escape by altering the immune microenvironment. In addition, fungal-derived metabolites and their interactions with host immune pathways can significantly modulate the efficacy of immunotherapies. These findings have spurred interest in exploring antifungal strategies as adjunctive approaches in cancer management, positioning antifungal therapy as a burgeoning area of oncological research. This review provides an in-depth exploration of the complex interplay between fungi and cancer. It examines the multifaceted role of fungal infections in tumor biology, the mechanisms through which fungi reshape the TME through immune modulation and their influence on immune-evasion strategies and therapeutic resistance. Furthermore, the potential for integrating antifungal therapies into comprehensive cancer treatment regimens has been highlighted, offering insights into novel avenues for improving patient outcomes.
Keywords: antifungal therapy; antifungal therapy fungi; cancer; fungal derived metabolites; fungal-derived metabolites; immunity; tumor microenvironment.
Copyright © 2025 Zhang, Zhang, Gao, Lei and Suo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Microbiome-mediated immune modulation in tumor microenvironment.Int Rev Cell Mol Biol. 2025;394:107-145. doi: 10.1016/bs.ircmb.2024.12.012. Epub 2025 Feb 17. Int Rev Cell Mol Biol. 2025. PMID: 40623764 Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Oncolytic virotherapy and tumor microenvironment modulation.Clin Exp Med. 2025 Jul 20;25(1):256. doi: 10.1007/s10238-025-01691-2. Clin Exp Med. 2025. PMID: 40685482 Free PMC article. Review.
-
Tumor microenvironment and immunotherapy: from bench to bedside.Med Oncol. 2025 Jun 8;42(7):244. doi: 10.1007/s12032-025-02818-x. Med Oncol. 2025. PMID: 40483667 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Alnuaimi A. D., Wiesenfeld D., O’Brien-Simpson N. M., Reynolds E. C., McCullough M. J. (2015). Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case-control study. Oncol. 51, 139–145. doi: 10.1016/j.oraloncology.2014.11.008 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

