Early Atrial Remodeling Drives Arrhythmia in Fabry Disease
- PMID: 40557493
- PMCID: PMC7617850
- DOI: 10.1161/CIRCEP.124.013352
Early Atrial Remodeling Drives Arrhythmia in Fabry Disease
Abstract
Background: Fabry disease (FD) is an X-linked lysosomal storage disorder caused by α-Gal A (α-galactosidase A) deficiency, resulting in multiorgan accumulation of sphingolipid, namely globotriaosylceramide. This triggers ventricular myocardial hypertrophy, fibrosis, and inflammation, driving arrhythmia and sudden death. Atrial fibrillation is common, yet the cellular mechanisms accounting for this are unknown.
Methods: To address this, we conducted ECG analysis from a large cohort of 115 adults with FD at varying cardiomyopathy stages. ECG P-wave characteristics were compared with non-FD controls. Cellular contractile and electrophysiological function were examined in a novel atrial cellular FD model developed and imputed into in silico atrial models to provide insight into mechanisms of arrhythmia. Induced pluripotent stem cells were genome-edited using Clustered Regularly Interspaced Short Palindromic Repeats-Cas9 to introduce the GLA p.N215S variant and differentiated into induced pluripotent stem cell-derived atrial cardiomyocytes (iPSC-CMs). Contraction, calcium handling, and electrophysiology experiments were conducted. Bi-atrial in silico models were developed with cellular changes as in GLA p.N215S iPSC-CMs.
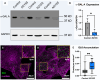
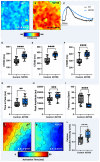
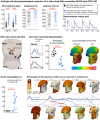
Results: ECG analysis demonstrated P-wave duration and PQ interval shortening in FD adults before the onset of cardiomyopathy. Patients with FD exhibited a higher incidence of premature atrial contractions and increased risk of atrial fibrillation compared with healthy controls. GLA p.N215S iPSC-CMs were deficient in α-Gal A and exhibited globotriaosylceramide accumulation. Atrial GLA p.N215S iPSC-CMs demonstrated a more positive diastolic membrane potential, faster action potential upstroke velocity, greater incidence of delayed afterdepolarizations, greater contraction force, and alterations in calcium handling compared with wild-type iPSC-CMs. Simulations with these changes in the in silico models resulted in similar P-wave morphology changes to those seen in early FD cardiomyopathy and increased atrial fibrillation vulnerability.
Conclusions: These findings provide novel insights into underpinning mechanisms for atrial arrhythmia and a rationale for early P-wave changes in FD. These may be targeted to develop therapeutic strategies to reduce the arrhythmic burden in FD.
Keywords: Fabry disease; adult; atrial fibrillation; electrocardiography; induced pluripotent stem cells.
Conflict of interest statement
None.
Figures




References
-
- Desnick RJ, Blieden LC, Sharp HL, Hofschire PJ, Moller JH. Cardiac valvular anomalies in Fabry disease. Clinical, morphologic, and biochemical studies. Circulation. 1976 Nov;54(5):818–25. - PubMed
-
- Desnick RJ, Wasserstein MP. Fabry disease: clinical features and recent advances in enzyme replacement therapy. Adv Nephrol Necker Hosp. 2001;31:317–39. - PubMed
-
- Mehta A, Beck M, Eyskens F, et al. Fabry disease: a review of current management strategies. QJM. 2010 Sep;103(9):641–59. - PubMed
-
- Biegstraaten M, Arngrímsson R, Barbey F, et al. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015 Mar 27;10:36. doi: 10.1186/s13023-015-0253-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

