Programmed death ligand-1 PET imaging in patients with melanoma: a pilot study
- PMID: 40557547
- PMCID: PMC12393058
- DOI: 10.1097/CMR.0000000000001050
Programmed death ligand-1 PET imaging in patients with melanoma: a pilot study
Abstract
Programmed death ligand-1 (PD-L1) is an inducible protein heterogeneously expressed in melanoma. Assessment of PD-L1 expression is challenging and standard immunohistochemistry (IHC) requires biopsies and cannot capture heterogeneity of expression. Noninvasive imaging methods provide evaluation of expression across lesions in the body. We conducted a prospective pilot trial with PD-L1 PET imaging with [ 18 F]-BMS-986229 as a noninvasive approach to assess PD-L1 expression across lesions, in 10 patients with advanced melanoma, longitudinally during treatment with nivolumab and ipilimumab. PET imaging was performed at baseline and at 6 weeks after initiation of treatment. We examined the relationship of PD-L1 PET uptake to radiographic clinical response. [ 18 F]-BMS-986229 uptake was variably seen across lesions in patients at baseline. All patients showed positive uptake in lesions at baseline PET with a median SUV max of 3.6 (range: 1.7-8.6). PD-L1 PET SUV max decreased in all but two lesions 6 weeks after treatment initiation. Four of five patients had a mean (SUV max ) greater than or equal to 3.00 in Response Evaluation Criteria in Solid Tumors (RECIST) evaluable lesions at baseline, and all had a RECIST response while all progressors ( n = 3) had baseline PD-L1 mean SUV max less than or equal to 2.60. A higher lesional baseline SUV max was associated with greater individual lesion reduction during treatment. The PD-L1 uptake in lesions showed a low correlation with baseline PD-L1 by IHC. In this small pilot study, PD-L1 PET imaging using [ 18 F]-BMS-986229 showed feasibility in noninvasively assessing lesion uptake and PD-L1 heterogeneity in patients receiving combination immunotherapy. Future exploration of this tracer in larger patient cohorts is necessary to delineate its use in managing immunotherapy treatments.
Trial registration: ClinicalTrials.gov NCT03122522.
Keywords: PD-L1; immunoPET; immunotherapy; melanoma.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.A.P. reports consulting fees from BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro, and Eisai, and institutional support from RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, and AstraZeneca. N.P.-T. has served as a consultant or advisory board member for, and received honoraria from, Actinium Pharma, Progenics, MedImmune/AstraZeneca, Telix Pharma, Cellectar Illumina, and ImaginAb, and conducts research institutionally supported by Y-mAbs Therapeutics, ImaginAb, Telix, Cellectar, innervate, Fusion Pharma, BMS, Bayer, Clarity Pharma, Janssen, and Regeneron. For the remaining authors, there are no conflicts of interest.
Figures
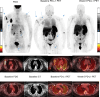


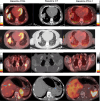
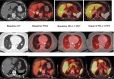
References
-
- Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 2015; 28:245–253. - PubMed
-
- Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 2018; 24:1852–1858. - PubMed
-
- Verhoeff S, van de Donk PP, Aarntzen E, Miedema IH, Oosting S, Voortman J, et al. 89Zr-durvalumab PD-L1 PET in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck. J Clin Oncol 2020; 38:3573–3573.
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

