Novabeads: Stimuli-Responsive Signal-Amplifying Hydrogel Microparticles for Enzymeless Fluorescence-Based Detection of microRNA Biomarkers
- PMID: 40557570
- PMCID: PMC12372440
- DOI: 10.1002/smll.202503990
Novabeads: Stimuli-Responsive Signal-Amplifying Hydrogel Microparticles for Enzymeless Fluorescence-Based Detection of microRNA Biomarkers
Abstract
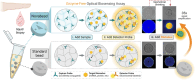
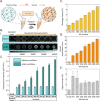
Robust and ultrasensitive biosensing platforms for detecting clinically relevant biomarkers from liquid biopsies are vital for precision diagnostics. However, detecting low-abundance biomarkers such as microRNA typically necessitates complex and costly enzyme-based strategies like PCR or isothermal amplification. Here, a materials-driven approach is leveraged to rationally design stimuli-responsive, signal-amplifying, and graphically-encoded hydrogel microparticles, termed Novabeads, for enzyme-free and fluorescence-based biomarker detection. Novabeads incorporate pH-responsive acrylic acid moieties within a polyethylene glycol diacrylate-based network, enabling significant volume reduction (≈5 fold) upon pH modulation. This stimuli-responsive shrinking, coupled with high bioreceptor loading via thiol-ene click chemistry, enables rapid, enzyme-free optical signal amplification. As a proof-of-concept, fluorescently-labeled peptide nucleic acid (PNA) probes are designed for detecting the cancer biomarker miR-16, via a fluorogenic Förster resonance energy transfer (FRET)-based signal. Novabeads exhibit >30 fold signal enhancement over equivalent conventional hydrogel microparticles, driven by three synergistic mechanisms: increased probe loading (≈2.6 fold), enhanced target capture (≈2.8 fold), and shrinkage-driven amplification (≈5 fold), ultimately leading to over 7 fold reduction in detection limit (28.8 pM; 2.9 fmol), and an expanded linear dynamic range. This rationally designed materials-driven biosensing strategy enables next-generation robust, versatile and enzyme-free biosensors for liquid biopsy diagnostics.
Keywords: biosensing; microRNA; peptide nucleic acid; smart hydrogel; stimuli‐responsive.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

