DWORF expression is reduced in a large animal model of Duchenne muscular dystrophy
- PMID: 40557917
- PMCID: PMC12233064
- DOI: 10.1242/dmm.052285
DWORF expression is reduced in a large animal model of Duchenne muscular dystrophy
Abstract
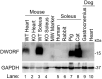
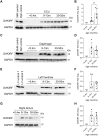
Duchenne muscular dystrophy (DMD) is a lethal muscle-wasting disease driven by cytosolic calcium overload, which leads to muscle degeneration. Sarco/endoplasmic reticulum calcium ATPase (SERCA), a key regulator of cytosolic calcium levels, exhibits reduced activity in animal models of DMD and human patients. Dwarf open reading frame (DWORF), a positive SERCA regulator, is downregulated in mdx DMD mice, and adeno-associated virus-mediated DWORF overexpression has been shown to ameliorate DMD cardiomyopathy. The canine DMD model provides a crucial bridge for translating findings from mice to humans. To investigate DWORF expression in this model, we developed a canine-specific anti-DWORF antibody, as the existing murine antibody is ineffective. This antibody detected DWORF in human, pig, cat and rabbit muscle, but not in mouse muscle. DWORF was absent in muscle tissues of neonatal normal dogs but highly expressed in those of adult dogs. In DMD-affected dogs aged 8 months or older, DWORF expression was significantly reduced in both cardiac and skeletal muscle. This study establishes a foundation for evaluating DWORF-based gene therapy in the canine DMD model, advancing the potential for clinical translation.
Keywords: DMD; Canine model; DWORF; Duchenne muscular dystrophy; SERCA2a.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests D.D. is a member of the scientific advisory board for Sardocor Corp, and an inventor of several issued and filed patents on DMD gene therapy and AAV vectors. R.W.H. is serving on scientific advisory boards for Regeneron Pharmaceuticals-Intellia Therapeutics collaboration, Prevail Therapeutics, Pfizer and Biomarin, and is also receiving funding from Roche. The other authors declare no competing or financial interests.
Figures



References
MeSH terms
Substances
Grants and funding
- R01 HL171221/HL/NHLBI NIH HHS/United States
- PRE1020028/American Heart Association
- MD210064/U.S. Department of Defense
- NS-131416/NH/NIH HHS/United States
- Jackson Freel DMD Research Fund
- R01 HL160569/HL/NHLBI NIH HHS/United States
- HL-160569/NH/NIH HHS/United States
- NS-090634/NH/NIH HHS/United States
- HL-171221/NH/NIH HHS/United States
- R01 AR070517/AR/NIAMS NIH HHS/United States
- R01 NS131416/NS/NINDS NIH HHS/United States
- AR-070517/NH/NIH HHS/United States
- R01 NS090634/NS/NINDS NIH HHS/United States
- AI-177600/NH/NIH HHS/United States
- University of Cincinnati
- R01 AI177600/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

