Deconstructing the Thymic Microenvironment Through Genesis to Senescence
- PMID: 40558001
- PMCID: PMC12188952
- DOI: 10.1111/imr.70048
Deconstructing the Thymic Microenvironment Through Genesis to Senescence
Abstract
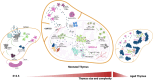
The thymus is essential for adaptive immunity, orchestrating the differentiation of hematopoietic progenitors into various T-cell lineages. Thymic epithelial cells (TECs) impart this unique function by mediating the major checkpoints in T-cell differentiation while also imposing stringent tolerance processes required to prevent autoimmunity. Achieving these feats requires extensive TEC specialization and the formation of distinct thymic microenvironments. These features change extensively throughout life, from the growth phases of the embryonic and perinatal thymus, into the steady-state adult, through responses to acute injury and regeneration and, finally, during age-related thymic involution. Here we review how hypothesis and technology have shaped the field's understanding of the thymic microenvironment. We focus on how the development of single-cell technologies has revealed a remarkably diverse cellular landscape shaped by progenitor cell differentiation, TEC proliferation, AIRE-mediated transcriptional processes, and the differentiation of thymic mimetic cell lineages.
Keywords: T‐cell differentiation; epithelium; single‐cell technologies; stromal cells; thymus; tolerance.
© 2025 The Author(s). Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The Walter and Eliza Hall Institute of Medical Research receives milestone and royalty payments related to venetoclax. Employees are entitled to receive benefits related to these payments; D.H.D.G. reports receiving benefits. D.H.D.G. has received research funding from Servier.
Figures
Similar articles
-
Cortical Thymic Epithelial Cells: Key Developers of the Code of T-Cell Selection and TCR Repertoire Diversity.Immunol Rev. 2025 Jul;332(1):e70049. doi: 10.1111/imr.70049. Immunol Rev. 2025. PMID: 40558027 Review.
-
Developmental trajectory and evolutionary origin of thymic mimetic cells.Nature. 2025 Jul;643(8073):1066-1075. doi: 10.1038/s41586-025-09148-y. Epub 2025 Jun 11. Nature. 2025. PMID: 40500437 Free PMC article.
-
RANKL treatment restores thymic function and improves T cell-mediated immune responses in aged mice.Sci Transl Med. 2024 Dec 4;16(776):eadp3171. doi: 10.1126/scitranslmed.adp3171. Epub 2024 Dec 4. Sci Transl Med. 2024. PMID: 39630886
-
Age-related epithelial defects limit thymic function and regeneration.Nat Immunol. 2024 Sep;25(9):1593-1606. doi: 10.1038/s41590-024-01915-9. Epub 2024 Aug 7. Nat Immunol. 2024. PMID: 39112630 Free PMC article.
-
Stem cell insights into human trophoblast lineage differentiation.Hum Reprod Update. 2016 Dec;23(1):77-103. doi: 10.1093/humupd/dmw026. Epub 2016 Sep 2. Hum Reprod Update. 2016. PMID: 27591247
References
-
- Miller J. F., “Immunological Function of the Thymus,” Lancet 2, no. 7205 (1961): 748–749. - PubMed
-
- Miller J. F., “Analysis of the Thymus Influence in Leukaemogenesis,” Nature 191 (1961): 248–249. - PubMed
-
- Miller J. F., “Effect of Neonatal Thymectomy on the Immunological Responsiveness of the Mouse,” Proceedings of the Royal Society of London, Series B: Biological Sciences 156 (1962): 415–428.
-
- Garcia K. C., “Dual Arms of Adaptive Immunity: Division of Labor and Collaboration Between B and T Cells,” Cell 179, no. 1 (2019): 3–7. - PubMed
-
- Ashby K. M. and Hogquist K. A., “A Guide to Thymic Selection of T Cells,” Nature Reviews. Immunology 24, no. 2 (2024): 103–117. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

