Regulatory T Cell in Kidney Transplant: The Future of Cell Therapy?
- PMID: 40558103
- PMCID: PMC12189525
- DOI: 10.3390/antib14020049
Regulatory T Cell in Kidney Transplant: The Future of Cell Therapy?
Abstract
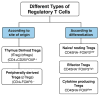
The long-term use of immunosuppressive drugs following kidney transplantation increases the risk of life-threatening infections, malignancies, and, paradoxically, eventual allograft rejection. Therefore, achieving a balance between over-immunosuppression and under-immunosuppression is critical to optimizing patient outcomes. One promising approach is immune cell-based therapy using suppressor immune cells to modulate the immune response more precisely. Among these, regulatory T cells (Tregs) are the most extensively studied and have shown significant potential in the post-transplant setting. Tregs are broadly categorized into thymus-derived and peripherally derived subsets. Physiologically, they play key roles in maintaining immune tolerance, including in autoimmune diseases and within the tumor microenvironment. Their immunosuppressive functions are mediated through both contact-dependent and contact-independent mechanisms. Studies investigating the use of Tregs following kidney transplantation have shown encouraging results. This review summarizes the biology of Tregs and highlights current evidence supporting their role in transplant immunotherapy.
Keywords: graft versus host disease; immunosuppressive therapy; kidney transplant; regulatory T cells; treg adoptive cell therapy.
Conflict of interest statement
The authors declare no conflicts of interest relevant to the content of this manuscript.
Figures
Similar articles
-
Synbiotics, prebiotics and probiotics for solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. Cochrane Database Syst Rev. 2022. PMID: 36126902 Free PMC article.
-
Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients.Cochrane Database Syst Rev. 2017 Jul 20;7(7):CD004756. doi: 10.1002/14651858.CD004756.pub4. Cochrane Database Syst Rev. 2017. PMID: 28731207 Free PMC article.
-
Hematopoietic cell-based and non-hematopoietic cell-based strategies for immune tolerance induction in living-donor renal transplantation: A systematic review.Transplant Rev (Orlando). 2023 Dec;37(4):100792. doi: 10.1016/j.trre.2023.100792. Epub 2023 Aug 19. Transplant Rev (Orlando). 2023. PMID: 37709652
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.Health Technol Assess. 2005 May;9(21):1-179, iii-iv. doi: 10.3310/hta9210. Health Technol Assess. 2005. PMID: 15899149
-
Regulatory T cell therapy is associated with distinct immune regulatory lymphocytic infiltrates in kidney transplants.Med. 2025 May 9;6(5):100561. doi: 10.1016/j.medj.2024.11.014. Epub 2024 Dec 27. Med. 2025. PMID: 39731908 Clinical Trial.
Cited by
-
Pharmacogenetics association with long-term clinical evolution in a kidney transplant patients cohort.Curr Res Pharmacol Drug Discov. 2025 Jul 24;9:100230. doi: 10.1016/j.crphar.2025.100230. eCollection 2025. Curr Res Pharmacol Drug Discov. 2025. PMID: 40761554 Free PMC article.
References
-
- United States Renal Data System . 2023 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD, USA: 2023.
-
- Rubinstein J., Toner K., Gross T., Wistinghausen B. Diagnosis and management of post-transplant lymphoproliferative disease following solid organ transplantation in children, adolescents, and young adults. Best Pract. Res. Clin. Haematol. 2023;36:101446. doi: 10.1016/j.beha.2023.101446. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources