Efficacy and Safety of P. hybridus Leaf Extract Ze 339 for the Treatment of Allergic Rhinitis
- PMID: 40558112
- PMCID: PMC12189244
- DOI: 10.3390/arm93030013
Efficacy and Safety of P. hybridus Leaf Extract Ze 339 for the Treatment of Allergic Rhinitis
Abstract
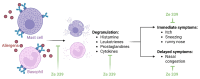
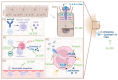
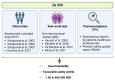
Allergic rhinitis (AR) is a global health problem on the rise. More and more people are affected, and climate change is exacerbating this health problem in the long term. The quality of life of those affected is often severely compromised, and the financial burden on healthcare systems cannot be disregarded. Therefore, effective and safe medicines are needed to counteract this trend. P. hybridus (butterbur) leaf extract (Ze 339) displays a promising alternative to antihistamines in the treatment of AR symptoms. More than two decades after the first market launch it is now possible to draw a meaningful conclusion on its safety and efficacy. This review summarizes the available preclinical and clinical data, real-world data (RWD) as well as data from post-marketing pharmacovigilance monitoring about the herbal medicinal drug Ze 339. It focusses on the current knowledge about the mode of action as well as the evaluation of its efficacy and safety in the treatment of AR. Given its favourable safety profile and lack of sedative side effects, Ze 339 offers a valuable alternative to antihistamines and should therefore continue to be considered by medical practitioners for the treatment of allergic rhinitis symptoms.
Keywords: Petasites hybridus; Ze 339; allergic rhinitis; butterbur; hay fever; safety.
Conflict of interest statement
V.M.M., G.B. and V.B. are employed by Max Zeller Söhne AG, the manufacturer of Ze 339. A.S. declares no conflict of interest.
Figures
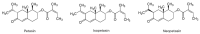
Similar articles
-
Herbal medicines for the treatment of allergic rhinitis: a systematic review.Ann Allergy Asthma Immunol. 2007 Dec;99(6):483-95. doi: 10.1016/S1081-1206(10)60375-4. Ann Allergy Asthma Immunol. 2007. PMID: 18219828
-
Herbal and dietary therapies for primary and secondary dysmenorrhoea.Cochrane Database Syst Rev. 2001;(3):CD002124. doi: 10.1002/14651858.CD002124. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2016 Mar 22;3:CD002124. doi: 10.1002/14651858.CD002124.pub2. PMID: 11687013 Updated.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Janeway C., Travers P., Walport M., Shlomchik M.J. Immunobiology: The Immune System in Health and Disease. 5th ed. Garland Science; New York, NY, USA: 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

