Efficacy of Endolysin LysAB1245 Combined with Colistin as Adjunctive Therapy Against Colistin-Resistant Gram-Negative Bacteria
- PMID: 40558128
- PMCID: PMC12189685
- DOI: 10.3390/antibiotics14060538
Efficacy of Endolysin LysAB1245 Combined with Colistin as Adjunctive Therapy Against Colistin-Resistant Gram-Negative Bacteria
Abstract
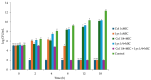
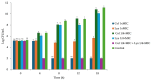
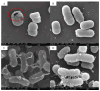
Background: Colistin resistance among Gram-negative nosocomial pathogens is an increasing concern. The bacteriophage-encoded lytic enzyme endolysin LysAB1245, which targets bacterial peptidoglycan, was evaluated as a potential antibacterial agent in combination with colistin as a therapeutic approach. Methods: Clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa, along with two reference strains, were used to assess the antibacterial activity of LysAB1245 and colistin, individually and in combination. Antibacterial susceptibility was assessed by broth microdilution. Synergistic interactions were determined using checkerboard assays and confirmed by time-kill kinetics. Resistance development was assessed after several rounds of exposure to each agent, either alone or in combination. Results: In this study, the synergistic activity of the LysAB1245/colistin combination therapy was found in some clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa, resulting in a reduction in the MICs of both LysAB1245 and colistin. The bactericidal effects, with a significant, more than 3-log reduction in CFU/mL (p < 0.01), were observed in representative synergistic isolates within 4 h of treatment with the combination of LysAB1245 at 1/4 × MIC and colistin at 1/4 × MIC. Scanning electron microscope micrographs confirmed bacterial cell damage upon treatment with the combination. Additionally, treatment with LysAB1245 in combination with colistin had no effect on the development of bacterial resistance after multiple passages. Conclusions: Combining LysAB1245 with a last-resort antibiotic like polymyxins (colistin) could be used as a promising new antibacterial strategy for preventing and controlling antibiotic-resistant Gram-negative bacteria.
Keywords: antibacterial agent; antibiotic-resistant bacteria; colistin; combination; endolysin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Enhancing colistin efficacy with combination therapies for multidrug-resistant P. aeruginosa and A. baumannii isolates.Future Microbiol. 2025 May-Jun;20(7-9):523-531. doi: 10.1080/17460913.2025.2490377. Epub 2025 Apr 10. Future Microbiol. 2025. PMID: 40208781
-
A "Time-Kill" Study to Assess the Effect of Colistin on Gram-Negative Strains: An Experimental Study for Developing an Indigenous Microfluidics-Based Antimicrobial Susceptibility Testing Device.Cureus. 2025 Jun 4;17(6):e85321. doi: 10.7759/cureus.85321. eCollection 2025 Jun. Cureus. 2025. PMID: 40621353 Free PMC article.
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Comparative Analysis of Colistin Susceptibility Using Micronaut MIC-Strip, Disc Elution, and Broth Microdilution in MDR Gram-Negatives.Clin Lab. 2025 Aug 1;71(8). doi: 10.7754/Clin.Lab.2024.241232. Clin Lab. 2025. PMID: 40779464
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Lepape A., Jean A., De Waele J., Friggeri A., Savey A., Vanhems P., Gustin M.P., Monnet D.L., Garnacho-Montero J., Kohlenberg A. European intensive care physicians’ experience of infections due to antibiotic-resistant bacteria. Antimicrob. Resist. Infect. Control. 2020;9:1. doi: 10.1186/s13756-019-0662-8. - DOI - PMC - PubMed
-
- Sader H.S., Mendes R.E., Streit J.M., Carvalhaes C.G., Castanheira M. Antimicrobial susceptibility of Gram-negative bacteria from intensive care unit and non-intensive care unit patients from United States hospitals (2018–2020) Diagn. Microbiol. Infect. Dis. 2022;102:115557. doi: 10.1016/j.diagmicrobio.2021.115557. - DOI - PubMed
-
- Salam M.T., Bari K.B., Rahman M.M., Gafur D.M.M., Faruk M.O., Akter K., Irin F., Ashakin M.R., Shaikat T.A., Das A.C., et al. Emergence of antibiotic-resistant infections in ICU patients. J. Angiother. 2024;8:1–9.
-
- Edwardson S., Cairns C. Nosocomial infections in the ICU. Anaesth. Intensive Care Med. 2019;20:14–18. doi: 10.1016/j.mpaic.2018.11.004. - DOI
-
- Lakbar I., Medam S., Ronflé R., Cassir N., Delamarre L., Hammad E., Lopez A., Lepape A., Machut A., Boucekine M., et al. Association between mortality and highly antimicrobial-resistant bacteria in intensive care unit-acquired pneumonia. Sci. Rep. 2021;11:16497. doi: 10.1038/s41598-021-95852-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

