Molecular and Phenotypic Evaluation of Antibiotic Resistance in Enteric Rods Isolated from the Oral Cavity
- PMID: 40558154
- PMCID: PMC12189819
- DOI: 10.3390/antibiotics14060564
Molecular and Phenotypic Evaluation of Antibiotic Resistance in Enteric Rods Isolated from the Oral Cavity
Abstract
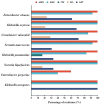
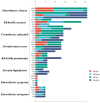
Gram-negative enteric rods (GNERs) are transient members of the oral microbiota and are considered a superinfection in patients with periodontitis that poses local and systemic risks due to associations with infections and multidrug resistance, including extended-spectrum beta-lactamases. These pathogens often resist antibiotics such as amoxicillin, doxycycline, and ciprofloxacin, complicating dental treatments. Though their resistance patterns vary, links between specific resistance genes and phenotypic resistance remain unclear. Objectives: To determine the correlation between resistance genes (blaTEM, blaSHV, tetQ, tetM, qnrB, qnrS, and mph(A)) and phenotypic resistance in GNERs isolated from oral cavity samples. Methods: A total of 90 oral isolates of GNERs were isolated from patients in a dental clinic, and bacteria were identified by the BD BBL Crystal biochemical panel. The antibiotic susceptibility testing was conducted through broth microdilution following CLSI standards for drives such as amoxicillin, amoxicillin/clavulanic acid, doxycycline, ciprofloxacin, and azithromycin. Resistance genes, including blaTEM, blaSHV, tetQ, tetM, qnrS, qnrB, and mph(A), were detected using polymerase chain reaction and gel electrophoresis. The proportions of species, resistance genes, and minimum inhibitory concentration values were statistically analyzed. Conclusions: As expected, most enteric bacteria showed natural resistance to beta-lactams. Significant resistance to azithromycin was observed in some species. Genotypic and phenotypic profiles suggest the existence of alternative resistance mechanisms; therefore, other mechanisms associated with antibiotic resistance should be investigated.
Keywords: antibiotic resistance; enteric rods; genes; oral cavity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Systemic antibiotics for chronic suppurative otitis media.Cochrane Database Syst Rev. 2021 Feb 4;2(2):CD013052. doi: 10.1002/14651858.CD013052.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2025 Jun 9;6:CD013052. doi: 10.1002/14651858.CD013052.pub3. PMID: 35819801 Free PMC article. Updated.
-
Molecular Characterization of Plasmid-Mediated Quinolone Resistance Genes in Multidrug-Resistant Escherichia coli Isolated From Wastewater Generated From the Hospital Environment.Environ Health Insights. 2025 Jun 22;19:11786302251342936. doi: 10.1177/11786302251342936. eCollection 2025. Environ Health Insights. 2025. PMID: 40552214 Free PMC article.
-
Interventions for treating genital Chlamydia trachomatis infection in pregnancy.Cochrane Database Syst Rev. 2017 Sep 22;9(9):CD010485. doi: 10.1002/14651858.CD010485.pub2. Cochrane Database Syst Rev. 2017. PMID: 28937705 Free PMC article.
-
Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever).Cochrane Database Syst Rev. 2011 Oct 5;2011(10):CD004530. doi: 10.1002/14651858.CD004530.pub4. Cochrane Database Syst Rev. 2011. PMID: 21975746 Free PMC article.
References
-
- Jepsen K., Falk W., Brune F., Cosgarea R., Fimmers R., Bekeredjian-Ding I., Jepsen S. Prevalence and antibiotic susceptibility trends of selected enterobacteriaceae, enterococci, and Candida albicans in the subgingival microbiota of German periodontitis patients: A retrospective surveillance study. Antibiotics. 2022;11:385. doi: 10.3390/antibiotics11030385. - DOI - PMC - PubMed
-
- Caton J.G., Armitage G., Berglundh T., Chapple I.L.C., Jepsen S., Kornman K.S., Mealey B.L., Papapanou P.N., Sanz M., Tonetti M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018;49:S1–S8. doi: 10.1002/jper.18-0157. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

