The Prevalence of Antimicrobial Resistance Genes in the Environments of Small Ruminant Farms from Central Portugal
- PMID: 40558166
- PMCID: PMC12189902
- DOI: 10.3390/antibiotics14060576
The Prevalence of Antimicrobial Resistance Genes in the Environments of Small Ruminant Farms from Central Portugal
Abstract
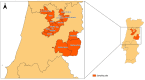
Background: Antimicrobial resistance is a pressing global concern affecting both human and animal health, with environment playing a key role in the dissemination of resistance determinants. This study aimed to investigate the presence of antimicrobial resistance genes (ARGs) associated with tetracyclines, β-lactams, macrolides, and sulfonamides in environmental matrices collected from 65 sheep and goat farms in central Portugal.
Methods: Environmental samples, including water, soil, pasture, and bedding, were analyzed through qPCR for the detection of clinically relevant ARGs.
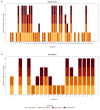
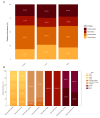
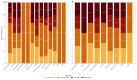
Results: ARGs were detected in 83% of the samples, with over half exhibiting genes from three or more antibiotic classes, suggesting potential multidrug resistance. β-lactamase genes were the most prevalent, followed by those conferring resistance to tetracycline and sulfonamide resistance, while macrolide resistance genes were least frequent. The distribution of ARGs varied by farm type, host species, and municipality.
Conclusions: These findings suggest that small ruminant farms serve as important reservoirs for ARGs. The results underscore the need for systematic surveillance and further research into the ecological and genetic factors driving ARG persistence and dissemination in extensive livestock systems, including proper waste management strategies to limit the spread and persistence of antibiotic resistance and mitigate broader public health risks.
Keywords: Portugal; antimicrobial resistance; goat; sheep.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control . Antimicrobial Resistance Surveillance in Europe 2022–2020 Data. WHO; Geneva, Switzerland: 2022.
Grants and funding
LinkOut - more resources
Full Text Sources

