Challenges of Carbapenem-Resistant Enterobacteriaceae in the Development of New β-Lactamase Inhibitors and Antibiotics
- PMID: 40558176
- PMCID: PMC12189368
- DOI: 10.3390/antibiotics14060587
Challenges of Carbapenem-Resistant Enterobacteriaceae in the Development of New β-Lactamase Inhibitors and Antibiotics
Abstract
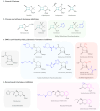
Nowadays, antimicrobial resistance (AMR) is a growing global health threat, with carbapenem-resistant Enterobacteriaceae (CRE) posing particular concern due to limited treatment options. In fact, CRE have been classified as a critical priority by the World Health Organization (WHO). Carbapenem resistance results from complex mechanisms, often combining the production of hydrolytic enzymes such as β-lactamases with reduced membrane permeability and efflux system induction. The Ambler classification is an effective tool for differentiating the characteristics of serine-β-lactamases (SβLs) and metallo-β-lactamases (MβLs), including ESβLs (different from carbapenemases), KPC, NDM, VIM, IMP, AmpC (different from carbapenemases), and OXA-48. Recently approved inhibitor drugs, such as diazabicyclooctanones and boronic acid derivatives, only partially address this problem, not least because of their ineffectiveness against MβLs. However, compared with taniborbactam, xeruborbactam is the first bicyclic boronate in clinical development with a pan-β-lactamase inhibition spectrum, including the IMP subfamily. Recent studies explore strategies such as chemical optimization of β-lactamase inhibitor scaffolds, novel β-lactam/β-lactamase inhibitor combinations, and siderophore-antibiotic conjugates to enhance bacterial uptake. A deeper understanding of the mechanistic properties of the active sites enables rational drug design principles to be established for inhibitors targeting both SβLs and MβLs. This review aims to provide a comprehensive overview of current therapeutic strategies and future perspectives for the development of carbapenemase inhibitor drug candidates.
Keywords: Enterobacteriaceae; boronic acid derivatives; carbapenems; diazabicyclooctanones; metallo-β-lactamases; serine-β-lactamases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Fleming A. Classics in Infectious Diseases: On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to Their Use in the Isolation of B. Influenzae by Alexander Fleming, Reprinted from the British Journal of Experimental Pathology 10:226–236, 1929. Rev. Infect. Dis. 1980;2:129–139. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

