Novel p-Hydroxybenzoic Acid Derivative Isolated from Bacopa procumbens and Its Antibacterial Activity
- PMID: 40558181
- PMCID: PMC12190013
- DOI: 10.3390/antibiotics14060591
Novel p-Hydroxybenzoic Acid Derivative Isolated from Bacopa procumbens and Its Antibacterial Activity
Abstract
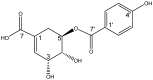
Background/Objectives: Antimicrobial resistance represents a critical global health challenge that has been exacerbated by the significant decline in antibiotic development. Natural product-based drugs, particularly plant-derived phenolic compounds, offer promising alternatives to conventional antibiotics. This study aimed to isolate and characterize a novel phenolic compound from Bacopa procumbens, a Mexican perennial repent plant that is widespread in the Mexican valley and produces a variety of saponins, gastrodin derivatives, and phenolic acids, and to evaluate its antibacterial potential against clinically relevant pathogens. Methods: The hydroalcoholic extraction of B. procumbens was followed by liquid-liquid partitioning with ethyl acetate. The resulting fraction underwent chromatographic separation and purification. The structural elucidation of the isolated compound was performed using thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), mass spectrometry (MS-EI), and nuclear magnetic resonance (NMR) techniques. Antimicrobial activity was assessed via a microdilution assay against five bacterial strains, including drug-resistant Staphylococcus species and Gram-negative pathogens. Results: A novel phenolic compound, 5-(p-hydroxybenzoyl) shikimic acid (5pHSA), was isolated and characterized. The compound demonstrated moderate antibacterial activity against methicillin-resistant Staphylococcus haemolyticus and Escherichia coli (minimum inhibitory concentration (MIC) = 100 μg/mL) but showed limited efficacy against Staphylococcus aureus, MRSA, and Klebsiella pneumoniae (MIC > 100 μg/mL). Comparative analysis with the previously isolated compound ProcumGastrodin A revealed structure-activity relationships where the higher lipophilicity of PG-A was correlated with enhanced antimicrobial activity. Conclusions: This study establishes 5pHSA as a novel phenolic compound with moderate antibacterial properties. The findings highlight the importance of molecular polarity and structural complexity in determining antimicrobial efficacy, offering valuable insights into the development of phenolic, acid-based antimicrobial agents to address the growing challenge of antimicrobial resistance.
Keywords: Bacopa procumbens; antimicrobial resistance; p-hydroxybenzoic acid derivatives; phenolic acids; plant natural products; shikimic acid derivatives.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- CDC . Antibiotic Resistance Threats in the United States 2019. CDC; Atlanta, GA, USA: 2019. AR Threats Report.
-
- World Health Organization (WHO) Antimicrobial Resistance. [(accessed on 13 April 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
Grants and funding
LinkOut - more resources
Full Text Sources

