Exploring Cancer Patients' and Caregivers' Perspectives and Knowledge Regarding Biomarker Testing in Canada
- PMID: 40558235
- PMCID: PMC12191459
- DOI: 10.3390/curroncol32060292
Exploring Cancer Patients' and Caregivers' Perspectives and Knowledge Regarding Biomarker Testing in Canada
Abstract
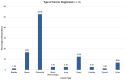
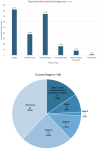
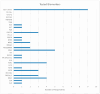
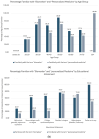
While biomarker testing can provide various benefits for cancer patient outcomes, numerous challenges persist that cause inequities in access across Canada. An online survey consisting of 51 questions was disseminated to evaluate biomarker testing and precision medicine knowledge and experiences from Canadian patients and caregivers. Responses were recorded between June 2023 and January 2024 and assessed various aspects of the biomarker testing experience including the expectations and challenges of patients. Quantitative and qualitative analyses were conducted using Microsoft Excel and R for descriptive and correlative data analysis, respectively. Among the 74 responses, patients reported an overall moderate experience with positive outcomes for those who underwent biomarker testing, including changes to treatment plans and the shrinking of tumours. The main challenges identified included knowledge gaps, a lack of testing availability, turnaround time for results, and financial constraints, all of which contribute to the disparities in biomarker testing access. Qualitative analysis of responses further emphasized a strong patient desire for patient-centred care and collaborative decision-making for biomarker testing options and treatment planning. Addressing these challenges through increased education, policy advocacy, and advancing infrastructure can help to reduce interprovincial inequities in biomarker testing and contribute to improving cancer patient outcomes.
Keywords: biomarker testing; cancer; cancer outcomes; colorectal cancer; genomic sequencing; patient-centred care; precision medicine; tumour.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Parents' and informal caregivers' views and experiences of communication about routine childhood vaccination: a synthesis of qualitative evidence.Cochrane Database Syst Rev. 2017 Feb 7;2(2):CD011787. doi: 10.1002/14651858.CD011787.pub2. Cochrane Database Syst Rev. 2017. PMID: 28169420 Free PMC article.
-
Interventions for patients and caregivers to improve knowledge of sickle cell disease and recognition of its related complications.Cochrane Database Syst Rev. 2016 Oct 6;10(10):CD011175. doi: 10.1002/14651858.CD011175.pub2. Cochrane Database Syst Rev. 2016. PMID: 27711980 Free PMC article.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review.Cochrane Database Syst Rev. 2018 Apr 17;4(4):CD010842. doi: 10.1002/14651858.CD010842.pub2. Cochrane Database Syst Rev. 2018. PMID: 29664187 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
References
-
- Canadian Cancer Statistics. [(accessed on 22 July 2024)]. Available online: https://cancer.ca/en/research/cancer-statistics/canadian-cancer-statistics.
-
- Definition of Biomarker. [(accessed on 20 September 2024)]; Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomarker.
-
- Biomarker Testing for Cancer Treatment. [(accessed on 22 September 2024)]; Available online: https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-ca....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

