Quality of Life of Lung Cancer Patients with Immune-Related Endocrinopathies During Immunotherapy: A Prospective Study Based on the EORTC QLQ-C30 and QLQ-LC13 Questionnaires in Romania
- PMID: 40558275
- PMCID: PMC12191735
- DOI: 10.3390/curroncol32060332
Quality of Life of Lung Cancer Patients with Immune-Related Endocrinopathies During Immunotherapy: A Prospective Study Based on the EORTC QLQ-C30 and QLQ-LC13 Questionnaires in Romania
Abstract
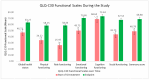
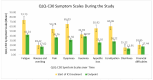
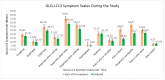
(1) Background: Globally, lung cancer is the leading cause of cancer death, but immunotherapy has impressively improved the outcomes, generating longer progression-free survival and overall survival. Endocrine immune-related adverse events (irAEs) are mostly irreversible and need life-long hormonal substitution therapy. The evaluation of the quality of life of lung cancer patients experiencing endocrine toxicities during immune checkpoint inhibitor (ICI) treatment is relevant for both patients and healthcare providers. (2) Methods: This was a prospective cohort study of lung cancer patients treated with immune checkpoint inhibitors in a tertiary-level hospital in Romania from 1 December 2021 to 30 September 2024. Quality of life was assessed using versions of the EORTC QLQ-C30 and EORTC QLQ-LC-13 validated and translated into the Romanian language. We investigated several clinical variables to evaluate their impact on QoL. (3) Results: Fifty-nine lung cancer patients were evaluated for their QoL before ICI initiation and at the end of the study. Endocrine-irAEs occurred in 17 lung cancer patients (28.8%). Quality of life as assessed using the EORTC questionnaires was statistically significantly improved, even in patients experiencing endocrine-irAEs. (4) Conclusions: Our prospective cohort study succeeded in delivering the proof of concept of an increased QoL in lung cancer patients who had developed endocrine-irAEs under immunotherapy. Despite toxicities, especially rather frequent endocrine-irAEs, ICIs enabled durable disease control and symptom relief, improving the QoL of the overall trial population. As more lung cancer patients undergo immunotherapy in metastatic or early stages, we draw attention to this particular patient population with autoimmune endocrinopathies, as they will live longer and require life-long hormonal therapy.
Keywords: Romania; endocrine immune-related adverse events; immune checkpoint inhibitors; lung cancer; quality of life questionnaire.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Immunotherapy for advanced or metastatic urothelial carcinoma.Cochrane Database Syst Rev. 2023 Oct 9;10(10):CD013774. doi: 10.1002/14651858.CD013774.pub2. Cochrane Database Syst Rev. 2023. PMID: 37811690 Free PMC article.
-
Real-world data of immune-related adverse events in lung cancer patients receiving immune-checkpoint inhibitors.Immunotherapy. 2025 Apr;17(5):321-329. doi: 10.1080/1750743X.2025.2488728. Epub 2025 Apr 4. Immunotherapy. 2025. PMID: 40183219
-
Immune Checkpoint Inhibitors-Induced Endocrinopathies: Assessment, Management and Monitoring in a Comprehensive Cancer Centre.Endocrinol Diabetes Metab. 2024 Jul;7(4):e00505. doi: 10.1002/edm2.505. Endocrinol Diabetes Metab. 2024. PMID: 38932429 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Global Cancer Observatory International Agency for Research on Cancer. [(accessed on 3 March 2025)]. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/642-roman....
-
- Remon J., Soria J.C., Peters S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021;32:1637–1642. doi: 10.1016/j.annonc.2021.08.1994. - DOI - PubMed
-
- Spigel D.R., Faivre-Finn C., Gray J.E., Vicente D., Planchard D., Paz-Ares L., Vansteenkiste J.F., Garassino M.C., Hui R., Quantin X., et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022;40:1301–1311. doi: 10.1200/JCO.21.01308. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

