Using Machine Learning Approaches on Dynamic Patient-Reported Outcomes to Cluster Cancer Treatment-Related Symptoms
- PMID: 40558277
- PMCID: PMC12191751
- DOI: 10.3390/curroncol32060334
Using Machine Learning Approaches on Dynamic Patient-Reported Outcomes to Cluster Cancer Treatment-Related Symptoms
Abstract
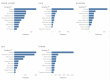
In patients undergoing systemic treatment for cancer, symptom tracking via electronic patient-reported outcomes (ePROs) has been used to optimize communication and monitoring, and facilitate the early detection of adverse effects and to compare the side effects of similar drugs. We aimed to examine whether the patterns in electronic patient-reported outcomes, without any additional clinician data input, are predictive of the underlying cancer type and reflect tumor- and treatment-associated symptom clusters (SCs). The data were derived from a total of 226 patients who self-reported on the presence and severity (according to the Common Terminology Criteria for Adverse Events (CTCAEs)) of more than 90 available symptoms via the mediduxTM app (versions 2.0 and 3.2, developed by mobile Health AG based in Zurich, Switzerland). Among these, 172 had breast cancer as the primary tumor, 19 had lung, 16 had gut, 12 had blood-lymph, and 7 had prostate cancer. For this secondary analysis, a subgroup of 25 patients with breast cancer were randomly selected to reduce the risk of overfitting. The symptoms were aggregated by counting the days on which a particular symptom was reported, resulting in a symptom vector for each patient. A logistic regression model was trained to predict the type of the respective tumor from the symptom vectors, and the symptoms with coefficients above (0.1) were graphically displayed. The machine learning model was not able to recognize any of the patients with prostate and blood-lymph cancer, likely as these cancer types were barely represented in the dataset. The Area Under the Curve (AUC) values for the three remaining cancer types were breast cancer: 0.74 (95% CI [0.624, 0.848]); gut cancer: 0.78 (95% CI [0.659, 0.893]); and lung cancer: 0.63 (95% CI [0.495, 0.771]). Despite the small datasets, for the breast and gut cancers, the respective models demonstrated a fair predictive performance (AUC > 0.7). The generalization of the findings are limited especially due to the heterogeneity of the dataset. This line of research could be especially interesting to monitor individual treatment trajectories. Deviations in the electronic patient-reported symptoms from the treatment-associated symptom patterns could dynamically indicate treatment non-adherence or lower treatment efficacy, without clinician input or additional costs. Similar analyses on larger patient cohorts are needed to validate these preliminary findings and to identify specific and robust treatment profiles.
Keywords: adherence; cancer; decision support; eHealth; electronic patient-reported outcome (ePRO); machine learning; real world evidence; symptom cluster.
Conflict of interest statement
A.T. is co-founder, stock owner and Chief Medical Officer of mobile Health AG, a start-up company that has developed, maintains and operates the mediduxTM platform. L.v.S. is the Scientific Project Manager of mobile Health AG.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Lee E.M., Jiménez-Fonseca P., Galán-Moral R., Coca-Membribes S., Fernández-Montes A., Sorribes E., García-Torralba E., Puntí-Brun L., Gil-Raga M., Cano-Cano J., et al. Toxicities and Quality of Life during Cancer Treatment in Advanced Solid Tumors. Curr. Oncol. 2023;30:9205–9216. doi: 10.3390/curroncol30100665. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical