Molecular Genetics of Renal Cell Carcinoma: A Narrative Review Focused on Clinical Relevance
- PMID: 40558302
- PMCID: PMC12192110
- DOI: 10.3390/curroncol32060359
Molecular Genetics of Renal Cell Carcinoma: A Narrative Review Focused on Clinical Relevance
Abstract
Molecular testing in renal cell carcinoma (RCC) has allowed for a better understanding of the biology of both sporadic and hereditary diseases, where genetic testing is currently recommended in the guidelines for a select population with risk factors. Historically, screening, surveillance, and management decisions were based solely on clinicopathologic data; however, we now know that molecular profiling can enhance decision making, altering the treatment plan, approach, or selection of systemic therapy and enhancing the delivery of precision oncologic care. Advances and the increasing availability of next-generation sequencing technologies have improved the identification of germline and somatic variants in key RCC-associated genes. Given the molecular heterogeneity of RCC, these modern methods can identify unique genetic events that occur in a single individual, allowing for distinction between a metachronous tumor from metastases. Separate four-tier systems have been proposed to categorize germline and somatic variants according to their clinical significance, which should be highlighted. Additionally, emerging technologies, such as liquid biopsy, show potential for enhancing precision oncology in RCC. With this said, challenges, such as variant interpretation, ethical considerations, and accessibility, persist. This review examines the molecularly defined RCC, genetic testing methodologies currently available, their current clinical applications, limitations, and future directions.
Keywords: circulating tumor DNA; germline variants; molecular genetics; next-generation sequencing; panel testing; renal cell carcinoma; somatic variants.
Conflict of interest statement
The authors declare no conflicts of interest related to this article.
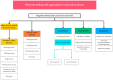
Figures
References
-
- Bratslavsky G., Mendhiratta N., Daneshvar M., Brugarolas J., Ball M.W., Metwalli A., Nathanson K.L., Pierorazio P.M., Boris R.S., Singer E.A., et al. Genetic risk assessment for hereditary renal cell carcinoma: Clinical consensus statement. Cancer. 2021;127:3957–3966. doi: 10.1002/cncr.33679. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

