Mettl7a alleviated bone loss in osteoporosis mice by targeting the O-GlcNAcylation of Bsp via m6A methylation
- PMID: 40558384
- PMCID: PMC12188527
- DOI: 10.1093/stcltm/szaf024
Mettl7a alleviated bone loss in osteoporosis mice by targeting the O-GlcNAcylation of Bsp via m6A methylation
Abstract
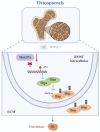
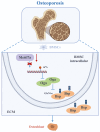
Postmenopausal osteoporosis, a prevalent metabolic bone disease, elevates susceptibility to fragility fractures while imposing substantial healthcare costs and public health challenges. The profound interplay between BMSCs and surrounding extracellular matrix (ECM) proteins, which are highly rich in O-GlcNAcylation, play pivotal roles in the process of osteoporosis. M6A methylation plays a crucial regulatory role in the development of osteoporosis, while the crosstalk between m6A methylation and ECM O-GlcNAcylation remains mechanistically undefined. Here we found Mettl7a overexpression improved the impaired osteogenic capability of OVX-mBMSCs in vitro. Conditional knockout of Mettl7a in the mesenchyme (Prx1-cre;Mettl7af/f) accelerated bone loss of OVX mice. Mechanistically, Mettl7a promoted mBMSCs osteogenic differentiation by targeting the O-GlcNAcylation of Bsp, an ECM protein. Mettl7a regulated the expression and O-GlcNAcylation of Bsp through m6A methylation of Oga. We further demonstrated that Mettl7a-AAV treatment alleviated bone loss phenotype in osteoporosis mice via the O-GlcNAcylation of Bsp. Collectively, our findings reveal novel mechanistic intersections between ECM protein O-GlcNAcylation and m6A methylation, advancing the understanding of osteoporotic regulation.
Keywords: Mettl7a; O-GlcNAcylation; extracellular matrix (ECM); m6A methylation; osteoporosis.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Crandall CJ, Larson J, Wright NC, et al. Serial bone density measurement and incident fracture risk discrimination in postmenopausal women. JAMA Intern Med. 2020;180:1232-1240. https://doi.org/ 10.1001/jamainternmed.2020.2986 - DOI - PMC - PubMed
-
- Yong EL, Logan S.. Menopausal osteoporosis: screening, prevention and treatment. Singapore Med J. 2021;62:159-166. https://doi.org/ 10.11622/smedj.2021036 - DOI - PMC - PubMed
-
- Barron RL, Oster G, Grauer A, Crittenden DB, Weycker D.. Determinants of imminent fracture risk in postmenopausal women with osteoporosis. Osteoporos Int. 2020;31:2103-2111. https://doi.org/ 10.1007/s00198-020-05294-3 - DOI - PMC - PubMed
-
- Compston JE, McClung MR, Leslie WD.. Osteoporosis. Lancet. 2019;393:364-376. https://doi.org/ 10.1016/S0140-6736(18)32112-3 - DOI - PubMed
-
- Reid IR, Billington EO.. Drug therapy for osteoporosis in older adults. Lancet. 2022;399:1080-1092. https://doi.org/ 10.1016/S0140-6736(21)02646-5 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

