Surface Plasmon Resonance Aptasensors: Emerging Design and Deployment Landscape
- PMID: 40558441
- PMCID: PMC12190323
- DOI: 10.3390/bios15060359
Surface Plasmon Resonance Aptasensors: Emerging Design and Deployment Landscape
Abstract
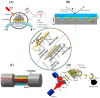
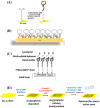
SPR biosensors operate on the principle of evanescent wave propagation at metal-dielectric interfaces in total internal reflection conditions, with consequent photonic energy attenuation. This plasmonic excitation occurs in specific conditions of incident light wavelength, angle, and the dielectric refractive index. This principle has been the basis for SPR-based biosensor setups wherein mass/concentration-induced changes in the refractive indices of dielectric media reflect as plasmonic resonance condition changes quantitatively reported as arbitrary response units. SPR biosensors operating on this conceptual framework have been designed to study biomolecular interactions with real-time readout and in label-free setups, providing key kinetic characterization that has been valuable in various applications. SPR biosensors often feature antibodies as target affinity probes. Notably, the operational challenges encountered with antibodies have led to the development of aptamers-oligonucleotide biomolecules rationally designed to adopt tertiary structures, enabling high affinity and specific binding to a wide range of targets. Aptamers have been extensively adopted in SPR biosensor setups with promising clinical and industrial prospects. In this paper, we explore the growing literature on SPR setups featuring aptamers, specifically providing expert commentary on the current state and future implications of these SPR aptasensors for drug discovery as well as disease diagnosis and monitoring.
Keywords: SELEX; aptamers; biosensors; drug discovery; ligands; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

