A Mobile Sperm Analyzer with User-Friendly Microfluidic Chips for Rapid On-Farm Semen Evaluation
- PMID: 40558476
- PMCID: PMC12190950
- DOI: 10.3390/bios15060394
A Mobile Sperm Analyzer with User-Friendly Microfluidic Chips for Rapid On-Farm Semen Evaluation
Abstract
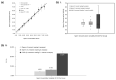
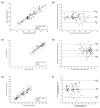
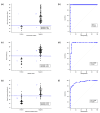
This study presents a mobile-based sperm analysis system featuring a user-friendly, droplet-loaded microfluidic chip that enables non-specialist users to perform the rapid and accurate quantitative evaluation of boar semen directly on the farm. The iSperm system integrates a tablet, optical module, heater, and real-time image analysis app to deliver automated measurements of sperm concentration, motility, and progressive motility in under one minute. Precision and user variability tests demonstrated high concordance with CASA and the hemocytometer, with minimal differences between trained and untrained users. A method comparison using 77 farm-collected samples confirmed agreement through Passing-Bablok regression and Bland-Altman analysis. ROC curve analyses further validated diagnostic accuracy for all parameters, with AUC values exceeding 0.95. The iSperm platform offers a reliable, user-friendly, and field-deployable solution for on-site semen quality assessment, improving decision-making in swine artificial insemination.
Keywords: ROC and regression analysis; boar semen analysis; image processing; microfluidic chip; on-farm point-of-care testing; portable sperm analysis system; user variability assessment.
Conflict of interest statement
S.L., C.C., C.L., T.C. and A.W. are inventors of iSperm technology and hold equity in Aidmics Biotechnology Ltd. Yu-Siang Tang and Chang-Ching Yeh were employed by Aidmics Biotechnology Co., Ltd., 11F.-1, No.171, Sec. 3, Roosevelt Rd., Da’an Dist., Taipei City 10647, Taiwan. Patent ownership belongs to National Taiwan University. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures






References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

