The Loss of Gonadal Hormones Has a Different Impact on Aging Female and Male Mice Submitted to Heart Failure-Inducing Metabolic Hypertensive Stress
- PMID: 40558497
- PMCID: PMC12190614
- DOI: 10.3390/cells14120870
The Loss of Gonadal Hormones Has a Different Impact on Aging Female and Male Mice Submitted to Heart Failure-Inducing Metabolic Hypertensive Stress
Abstract
Background: Aging and the female sex are considered risk factors for the development of heart failure with preserved ejection fraction (HFpEF). Unlike other risk factors, such as hypertension, obesity, or diabetes, they do not represent therapeutic targets.
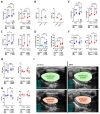
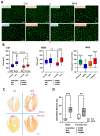
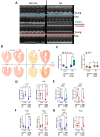
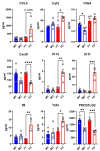
Methods: In a recently developed two-hit murine HFpEF model (angiotensin II + high-fat diet; MHS), we studied the relative contributions of the biological sex, aging, and gonadal hormones to cardiac remodeling and function. We aimed to reproduce a frequent HFpEF phenotype in mice characterized by aging, hypertension, the female sex, menopause, and metabolic alterations. Using the MHS mouse model, we studied cardiac remodeling and function in C57Bl6/J mice of both sexes, young (12 weeks) and old (20 months), that were gonadectomized (Gx) or not.
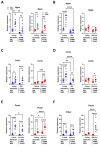
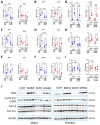
Results: We observed that in mice, aging was associated with body weight gain, cardiac hypertrophy (CH), left ventricle (LV) concentric remodeling, and left atrial (LA) enlargement. Diastolic parameters such as E and A wave velocities were modulated by aging but only in females. Submitting young and old mice to MHS for 28 days induced the expected HFpEF phenotype consisting of CH, LV wall thickening, LA enlargement, and diastolic dysfunction with a preserved EF except for old males, in which it was significantly reduced. Young mice were Gx at five weeks, and old mice at six months (over a year before MHS). Gx increased myocardial fibrosis in MHS females and helped preserve the EF in males.
Conclusions: Our results suggest that MHS has sex-specific effects on old mice, and the loss of gonadal hormones significantly impacts the observed heart failure phenotype.
Keywords: HFpEF; aging; cardiac hypertrophy; heart failure; mouse; preclinical model; sex differences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Radakrishnan A., Agrawal S., Singh N., Barbieri A., Shaw L.J., Gulati M., Lala A. Underpinnings of Heart Failure With Preserved Ejection Fraction in Women—From Prevention to Improving Function. A Co-publication With the American Journal of Preventive Cardiology and the Journal of Cardiac Failure. J. Card. Fail. 2025 doi: 10.1016/j.cardfail.2025.01.008. in press . - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

