Inorganic Arsenite [As (III)] Represses Human Renal Progenitor Cell Characteristics and Induces Neoplastic-like Transformation
- PMID: 40558504
- PMCID: PMC12191282
- DOI: 10.3390/cells14120877
Inorganic Arsenite [As (III)] Represses Human Renal Progenitor Cell Characteristics and Induces Neoplastic-like Transformation
Abstract
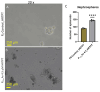
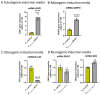
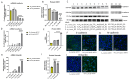
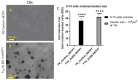
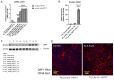
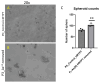
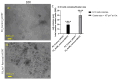
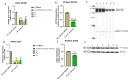
Arsenic, in the form of inorganic arsenite, is toxic to the kidney and can cause acute kidney injury, manifesting as destruction of proximal tubule cells. Nephron repair is possible through the proliferation of resident tubular progenitor cells expressing CD133 and CD24 surface markers. We simulated regenerative repair in the continued presence of i-As (III) using a cell culture model of a renal progenitor cell line expressing CD133 (PROM1) and CD24. Continued exposure and subculturing of progenitor cells to i-As (III) led to a reduction in the expression of PROM1 and CD24, as well as a decrease in the ability to differentiate into tubule-like structures. Cessation of i-As (III) and recovery for up to three passages resulted in continued repression of PROM1 and reduced ability to differentiate. Chronically exposed cells exhibited an ability to form colonies in soft agar, suggesting neoplastic transformation. Chronically exposed cells also exhibited an induction of CD44, a cell surface marker commonly found in renal cell carcinoma, as well as in tubular repair in chronic renal injury such as chronic kidney disease. These results demonstrate potential adverse outcomes of renal progenitor cells chronically exposed to a nephrotoxicant, as well as in environmental exposure to arsenic.
Keywords: CD44; HRTPT; RCC; arsenite; kidney progenitor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Chronic Exposure of Renal Progenitor Cells (HRTPT) to As (III) Implicates Microfibril Associated Protein 5 (MFAP5) in the Activation of Carcinoembryonic Antigen Related Cell Adhesion Molecules (CEACAM 5 and 6).Curr Issues Mol Biol. 2025 Jun 12;47(6):455. doi: 10.3390/cimb47060455. Curr Issues Mol Biol. 2025. PMID: 40699854 Free PMC article.
-
Identification of CD44+CD24+ gastric cancer stem cells.J Cancer Res Clin Oncol. 2011 Nov;137(11):1679-86. doi: 10.1007/s00432-011-1038-5. Epub 2011 Sep 1. J Cancer Res Clin Oncol. 2011. PMID: 21882047 Free PMC article.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article.
-
Chronic exposure of renal stem cells to inorganic arsenic induces a cancer phenotype.Chem Res Toxicol. 2013 Jan 18;26(1):96-105. doi: 10.1021/tx3004054. Epub 2012 Nov 19. Chem Res Toxicol. 2013. PMID: 23137061 Free PMC article.
-
Clinicopathological and prognostic significance of cancer stem cell markers CD44 and CD133 in patients with gastric cancer: A comprehensive meta-analysis with 4729 patients involved.Medicine (Baltimore). 2016 Oct;95(42):e5163. doi: 10.1097/MD.0000000000005163. Medicine (Baltimore). 2016. PMID: 27759647 Free PMC article.
References
-
- Fatoki J.O., Badmus J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022;5:100052. doi: 10.1016/j.hazadv.2022.100052. - DOI
-
- Nordstrom D.K. An Overview of Arsenic Mass-Poisoning in Bangladesh and West Bengal, India. Society for Mining, Metallurgy, and Exploration; Englewood, CO, USA: 2000. [(accessed on 25 January 2025)]. Available online: https://pubs.usgs.gov/publication/70198882.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

