Wnt/PKC Signaling Inhibits Sensory Hair Cell Formation in the Developing Mammalian Cochlea
- PMID: 40558515
- PMCID: PMC12191204
- DOI: 10.3390/cells14120888
Wnt/PKC Signaling Inhibits Sensory Hair Cell Formation in the Developing Mammalian Cochlea
Abstract
The establishment of cell fate and boundaries between cell types is an essential step in development and organogenesis. In the mammalian cochlea, a distinct boundary exists between a medial region of non-sensory cells and a lateral region of sensory cells. We report that Wnt4 and sFRP2 act in combination to modulate the sensory cell differentiation of the organ of Corti. The hair cell inhibitory effects of Wnt4 in the inner ear are mediated through the activation of the non-canonical Wnt/Calcium/PKC pathway. We show that Wnt4 stimulates the activation of PKC in the cochlea, and that the inhibition of PKC rescues the ectopic Wnt4 activity phenotype. Finally, we demonstrate that modification at a PKC target site on Atoh1 diminishes its ability to induce hair cell formation. Ultimately, we identify a new Wnt/Calcium/PKC non-canonical signaling pathway that is involved in proper hair cell and organ of Corti formation in the developing mammalian cochlea.
Keywords: cochlea; genes; hair cell; hearing; mouse.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
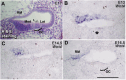
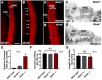
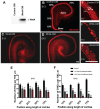
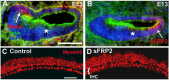
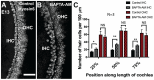
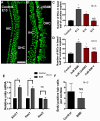
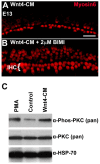
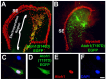
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

