Ectopic ULBP2 Is Associated with Decreased NKG2D Expression in CD8+ T Cells Under T Cell-Modulatory Conditions in a Murine Tumor Model
- PMID: 40558520
- PMCID: PMC12191310
- DOI: 10.3390/cells14120893
Ectopic ULBP2 Is Associated with Decreased NKG2D Expression in CD8+ T Cells Under T Cell-Modulatory Conditions in a Murine Tumor Model
Abstract
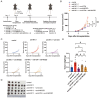
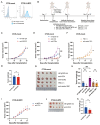
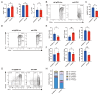
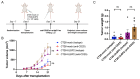
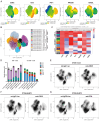
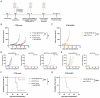
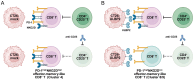
UL16-binding protein 2 (ULBP2), a ligand for the activating receptor NKG2D, plays a dual role in tumor immunity, promoting immune activation or suppression, depending on the context. To investigate its impact on CD4+CD25+ T cell-targeted immunotherapies, we used a syngeneic CT26 colon cancer model engineered to express ULBP2 and compared tumor growth and tumor-infiltrating lymphocyte (TIL) profiles in control and ULBP2-expressing tumors treated with anti-CD4, anti-CD25, or anti-CTLA-4 antibodies. Tumor growth was uniformly assessed on day 21 post-transplantation, and TIL analysis was performed in groups with evaluable residual tumors. Anti-CD4 antibody significantly suppressed tumor growth in mock-transfected tumors, while no significant suppression was observed in ULBP2-expressing tumors. Anti-CD25 antibody had limited efficacy in mock tumors and tended to promote tumor growth in ULBP2-expressing tumors. Following these treatments, ULBP2 expression was associated with reduced NKG2D expression in CD8+ effector memory T cells, particularly PD-1high subsets. In contrast, anti-CTLA-4 antibody treatment induced marked tumor regression irrespective of ULBP2 expression. These findings suggest that ULBP2-NKG2D signaling may contribute to altered CD8+ T cell phenotypes under T cell-modulatory conditions, potentially impacting the outcome of CD4+CD25+ T cell-targeted therapies and providing insights for optimizing immunotherapeutic strategies.
Keywords: CD4+ T cell depletion; CD4+ T cells; NK cells; NKG2D; NKG2D ligands; Treg; ULBP2; cancer immunotherapy; immune checkpoint inhibitor; tumor immunology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Different tumour-resident memory T-cell subsets regulate responses to anti-PD-1 and anti-CTLA-4 cancer immunotherapies.Nat Commun. 2025 Jul 1;16(1):5588. doi: 10.1038/s41467-025-60657-w. Nat Commun. 2025. PMID: 40593647 Free PMC article.
-
A fc-engineered NKG2D × B7-H3 bispecific antibody enhances the antitumor activity by orchestrating cytotoxic lymphocytes.Int Immunopharmacol. 2025 Aug 28;161:115032. doi: 10.1016/j.intimp.2025.115032. Epub 2025 Jun 13. Int Immunopharmacol. 2025. PMID: 40516256
-
ULBP2 Promotes Tumor Progression by Suppressing NKG2D-Mediated Anti-Tumor Immunity.Int J Mol Sci. 2025 Mar 24;26(7):2950. doi: 10.3390/ijms26072950. Int J Mol Sci. 2025. PMID: 40243581 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review.JAMA Oncol. 2016 Oct 1;2(10):1354-1360. doi: 10.1001/jamaoncol.2016.1061. JAMA Oncol. 2016. PMID: 27355489
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

