Autophagy and Alzheimer's Disease: Mechanisms and Impact Beyond the Brain
- PMID: 40558538
- PMCID: PMC12191281
- DOI: 10.3390/cells14120911
Autophagy and Alzheimer's Disease: Mechanisms and Impact Beyond the Brain
Abstract
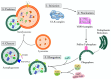
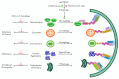
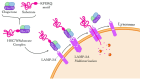
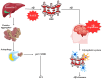
Alzheimer's disease (AD) is a progressive neurodegenerative disorder marked by neuronal loss, cognitive decline, and pathological hallmarks such as amyloid-beta (Aβ) plaques and tau neurofibrillary tangles. Recent evidence highlights autophagy as a pivotal mechanism in cellular homeostasis, mediating the clearance of misfolded proteins and damaged organelles. However, impaired autophagy contributes significantly to AD pathogenesis by disrupting proteostasis, exacerbating neuroinflammation, and promoting synaptic dysfunction. This review aims to scrutinize the intricate relationship between autophagy dysfunction and AD progression, explaining key pathways including macroautophagy, chaperone-mediated autophagy (CMA), and selective autophagy processes such as mitophagy and aggrephagy. This further extends the discussion beyond the central nervous system, evaluating the role of hepatic autophagy in Aβ clearance and systemic metabolic regulation. An understanding of autophagy's involvement in AD pathology via various mechanisms could give rise to a novel therapeutic strategy targeting autophagic modulation to mitigate disease progression in the future.
Keywords: Alzheimer’s disease; amyloid-beta clearance; autophagy; neurodegeneration; tau pathology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Vishnumukkala T., Gopalakrishna P.K., Jagadeesan S., Chiroma S.M., Mohd Nor N.H., Baharuldin M.T.H., Thomas W., Mohd Moklas M.A. Herbal Medicine: A Promising Approach for the Treatment of Alzheimer’s Disease. Int. J. Ayurveda Pharma Res. 2024;12:142–150. doi: 10.47070/ijapr.v12i1.3103. - DOI
-
- Liu Y., Wu Y., Chen Y., Lobanov-Rostovsky S., Liu Y., Zeng M., Bandosz P., Xu D.R., Wang X., Liu Y., et al. Projection for Dementia Burden in China to 2050: A Macro-Simulation Study by Scenarios of Dementia Incidence Trends. Lancet Reg. Health West. Pac. 2024;50:101158. doi: 10.1016/j.lanwpc.2024.101158. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

