Downregulated ALDH2 Contributes to Tumor Progression and Targeted Therapy Resistance in Human Metastatic Melanoma Cells
- PMID: 40558540
- PMCID: PMC12191128
- DOI: 10.3390/cells14120913
Downregulated ALDH2 Contributes to Tumor Progression and Targeted Therapy Resistance in Human Metastatic Melanoma Cells
Abstract
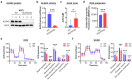
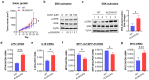
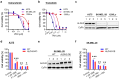
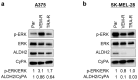
Aldehyde dehydrogenase 2 (ALDH2) is a crucial detoxifying enzyme that eliminates toxic aldehydes. ALDH2 deficiency has been linked to various human diseases, including certain cancers. We have previously reported ALDH2 downregulation in human melanoma tissues. Here, we further investigated the biological significance of ALDH2 downregulation in this malignancy. Analysis of TCGA dataset revealed that low ALDH2 expression correlates with poorer survival in metastatic melanoma. Examination of human metastatic melanoma cell lines confirmed that most had ALDH2 downregulation (ALDH2-low) compared to primary melanocytes. In contrast, a small subset of metastatic melanoma cell lines exhibited normal ALDH2 levels (ALDH2-normal). CRISPR/Cas9-mediated ALDH2 knockout in ALDH2-normal A375 cells promoted tumor growth and MAPK/ERK activation. Given the pivotal role of MAPK/ERK signaling in melanoma and cellular response to acetaldehyde, we compared A375 with ALDH2-low SK-MEL-28 and 1205Lu cells. ALDH2-low cells were intrinsically resistant to BRAF and MEK inhibitors, whereas A375 cells were not. However, A375 cells acquired resistance upon ALDH2 knockout. Furthermore, melanoma cells with acquired resistance to these inhibitors displayed further ALDH2 downregulation. Our findings indicate that ALDH2 downregulation contributes to melanoma progression and therapy resistance in BRAF-mutated human metastatic melanoma cells, highlighting ALDH2 as a potential prognostic marker and therapeutic target in metastatic melanoma.
Keywords: MAPK/ERK; acetaldehyde; aldehyde dehydrogenase 2; drug resistance; melanoma; targeted therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Koppaka V., Thompson D.C., Chen Y., Ellermann M., Nicolaou K.C., Juvonen R.O., Petersen D., Deitrich R.A., Hurley T.D., Vasiliou V. Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012;64:520–539. doi: 10.1124/pr.111.005538. - DOI - PMC - PubMed
-
- Maiuolo J., Oppedisano F., Carresi C., Gliozzi M., Musolino V., Macri R., Scarano F., Coppoletta A., Cardamone A., Bosco F., et al. The Generation of Nitric Oxide from Aldehyde Dehydrogenase-2: The Role of Dietary Nitrates and Their Implication in Cardiovascular Disease Management. Int. J. Mol. Sci. 2022;23:15454. doi: 10.3390/ijms232415454. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

