Hand Function Recovers to Near Normal in Patients with Deep Dermal Hand Burns Treated with Enzymatic Debridement: A Prospective Cohort Study
- PMID: 40558631
- PMCID: PMC12192170
- DOI: 10.3390/ebj6020036
Hand Function Recovers to Near Normal in Patients with Deep Dermal Hand Burns Treated with Enzymatic Debridement: A Prospective Cohort Study
Abstract
Short- and long-term hand function was evaluated in adult patients with deep dermal and full-thickness hand burns after treatment with enzymatic debridement (NexoBrid® MediWound Ltd., Yavne, Israel), assessing the results at discharge and 3, 6, and 12 months post-burn. This prospective cohort study was performed in the Burn Center in Beverwijk between March 2017 and December 2019. Hand function was assessed using Modified Kapandji Index scores, the Jebsen-Taylor Hand Function Test, and range of motion; scar quality using the Patient and Observer Scar Assessment Scale version 2.0; and quality of life using the Quick Disability Arm Shoulder Hand Questionnaire and the Canadian Occupational Performance Measure. Ten patients (14 hand burns) were included. The need for a skin graft after NexoBrid® was 86%, and 50% needed additional surgical excision before skin grafting. Digits 3 and 4 achieved near-to-normal total active motion, and at least 50% of the hands achieved a normal range within the Jebsen-Taylor Hand Function Test in four items at 12 months post-burn. Scar quality and quality of life improved significantly over time. The present study can be considered as a proof-of-concept study for future clinical trials on enzymatic debridement for hand burns.
Keywords: burns; enzymatic debridement; hand function; quality of life; scar quality.
Conflict of interest statement
Roelf S. Breederveld is the principal investigator at the local site (Red Cross Hospital, Beverwijk) that participated in the CIDS study (MW 2012-01-01), which is a multicenter, multinational, randomized, controlled, open-label study, performed in children with thermal burns to evaluate the efficacy and safety of NexoBrid compared to standard-of-care treatment. The CIDS study was initiated and is funded by Mediwound (Yavne, Israel), which is the manufacturer of NexoBrid. Kelly A. A. Kwa was a PhD candidate, not paid through the finances/grant received by Mediwound, who worked as a (local) researcher on the CIDS study. Prof. Paul P. M. van Zuijlen is the developer of the Patient and Observer Scar Assessment Scale.
Figures

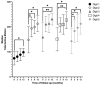
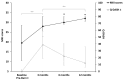
 ** significant for Q-DASH; p < 0.017.
** significant for Q-DASH; p < 0.017.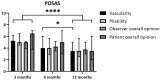
Similar articles
-
Evaluating scar outcomes in pediatric burn patients following skin grafting.Sci Rep. 2025 Jun 20;15(1):20205. doi: 10.1038/s41598-025-06378-y. Sci Rep. 2025. PMID: 40542136 Free PMC article.
-
Guided tissue regeneration for periodontal infra-bony defects.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001724. doi: 10.1002/14651858.CD001724.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2019 May 29;5:CD001724. doi: 10.1002/14651858.CD001724.pub3. PMID: 16625546 Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Interventions for necrotizing soft tissue infections in adults.Cochrane Database Syst Rev. 2018 May 31;5(5):CD011680. doi: 10.1002/14651858.CD011680.pub2. Cochrane Database Syst Rev. 2018. PMID: 29851032 Free PMC article.
References
-
- Bain C.J., Wang T., McArthur G., Williams G., Atkins J., Jones I. Safety and efficacy of excision and direct closure in acute burns surgery: Outcome analysis in a prospective series of 100 patients and a survey of UK burns surgeons’ attitudes. Burns. 2014;40:1635–1641. doi: 10.1016/j.burns.2014.02.021. - DOI - PubMed
-
- Muller M., Gahankari D., Herndon D.N. Operative Wound Management. In: Greenwood G., editor. Total Burn Care. 3rd ed. Philadelphia Saunders Elsevier; Beijing, China: 2007. pp. 177–195.
LinkOut - more resources
Full Text Sources
